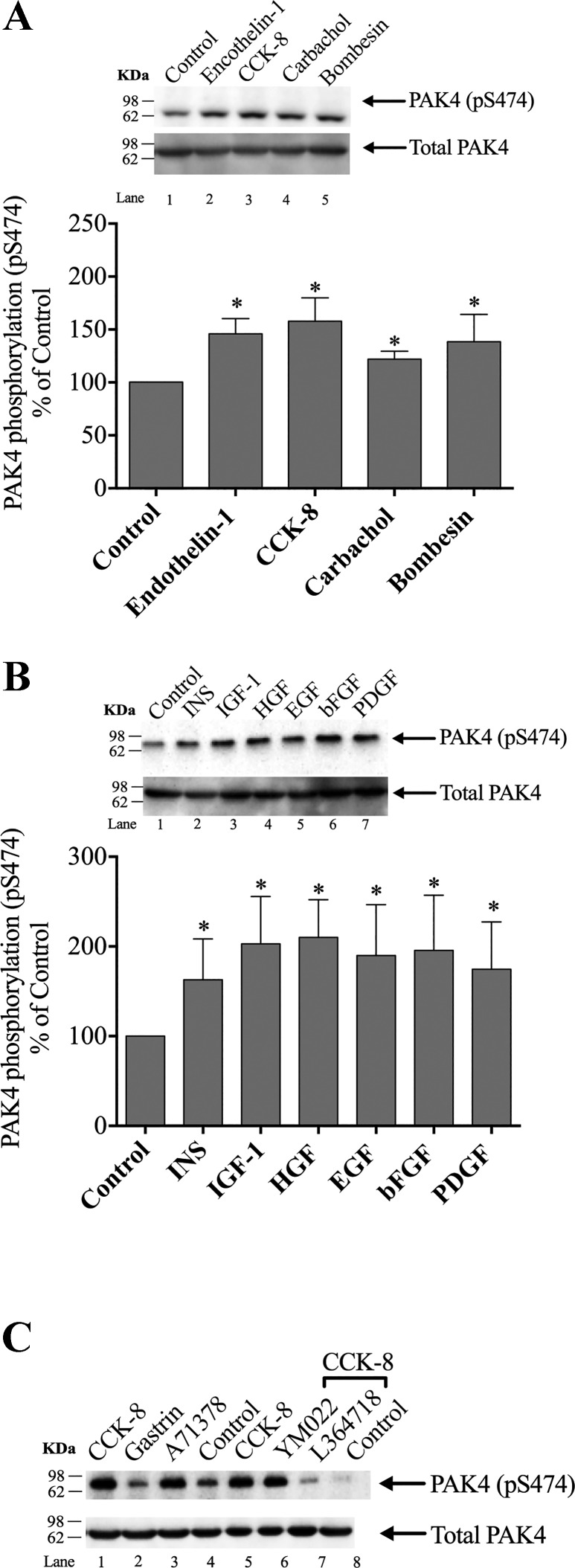
Fig. 2.
Ability of various pancreatic hormones/neurotransmitters and pancreatic growth factors to stimulate p21-activated kinase (PAK)4 phosphorylation (pS474) in rat pancreatic acini. A: ability of cholecystokinin (CCK)-8, carbachol, bombesin, or endothelin-1 to activate pS474 PAK4 in isolated pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of CCK-8 (1 nM), carbachol (10 µM), bombesin (1 nM), or endothelin-1 (10 nM) for 1 min and then lysed. Results are expressed as %control phosphorylation. B: ability of insulin (INS), insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF) to activate PAK4 in the pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of insulin (INS) (1 µM, 10 min), EGF (10 nM, 5 min), PDGF (100 ng/ml, 10 min), bFGF (100 ng/ml, 5 min), IGF (100 nM, 10 min), and HGF (1 nM, 10 min), and then lysed. Results expressed in A. C: representative Western blot assessing the ability of selective CCK1 or CCK2 receptor agonist/antagonists to alter PAK4 activation in pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of CCK-8 (1 nM); the CCK2 agonist, gastrin (1 nM); or the CCK1 receptor agonist, A71378 (30 nM) for 1 min; or preincubated for 5 min in the presence of the CCK2 antagonist, YM022 (1 µM); the CCK1 antagonist, L-364,718(1 µM); and then after the additional presence of CCK-8 (1 nM) for 3 min. The cell lysates were subjected to Western blotting and analyzed using anti-pS474 PAK4 and, as loading control, anti-total PAK4. Bands were visualized using chemiluminescence and quantified by densitometry. A and B, top: results of a representative blot of six independent experiments. Bottom of A and B: mean ± SE of six independent experiments. *P < 0.05 compared with the control.