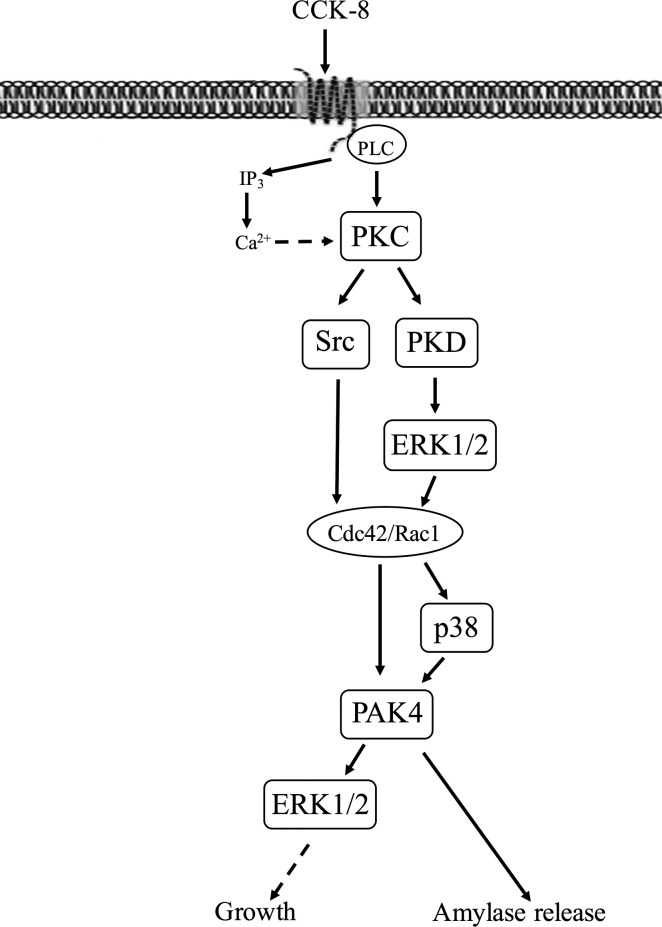
Fig. 13.
Schematic diagram of signaling cascade for activation of p21-activated kinase (PAK)4 in pancreatic acinar cells. In rat pancreatic acinar cells, maximal activation of PAK4 by cholecystokinin (CCK)-8 requires activation of primarily PKC, with a lesser contribution by changes in cytosolic calcium. PKC mediates both Src family of kinase (SFK) and protein kinase D (PKD) activation with the latter resulting in ERK1/2 activation and cell division control protein 42 homolog (Cdc42)/ Ras-related C3 botulinum toxin substrate 1 (Rac1) activation. This in turn can stimulate PAK4 activation partially via a p38 mechanism. PAK4 activation is important for CCK-stimulated enzyme secretion as well as ERK1/2 activation which has been shown to mediate growth (20, 30). However, changes in phosphatidylinositol 3-kinase (PI3K) are not involved but were reported in a number of other tissues (23, 80). The ERK1/2 and PAK4 inhibition studies demonstrate CCK-8-mediated ERK1/2 activation and PAK4 activation reciprocally regulate each other’s activation.