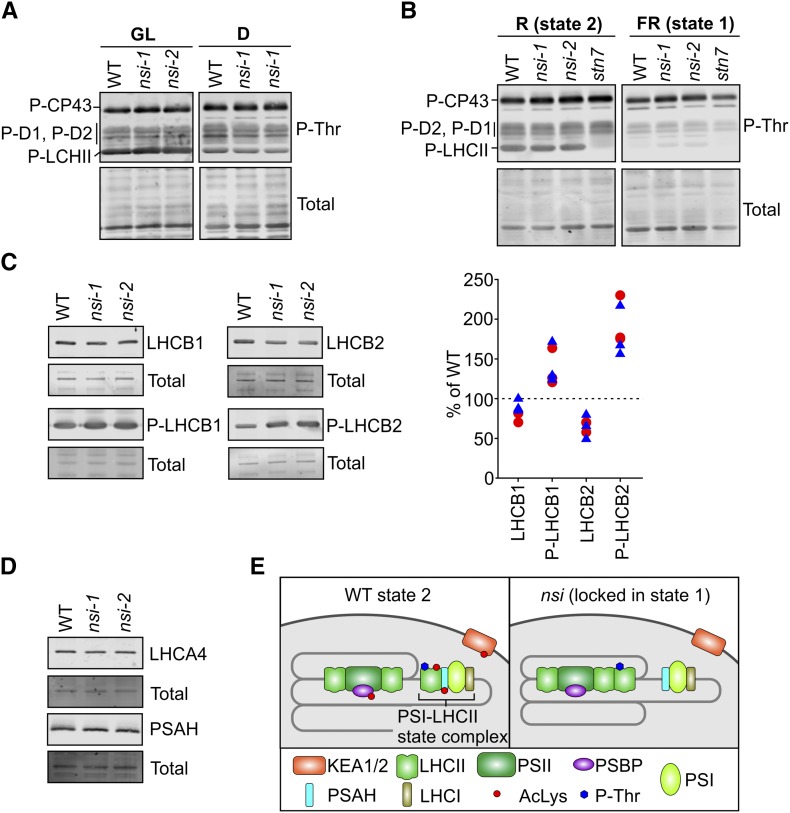
Figure 5.
Immunoblot Analysis of Thylakoid Protein Phosphorylation, L-LHCII Subunits, and PSI Docking Site for L-LHCII and Schematic Presentation of the Downregulated Lys Acetylation Sites in nsi.
(A) Phosphorylation of thylakoid proteins isolated from GL- or D-adapted plants. Proteins were separated on 15% acrylamide gels and immunoblotted with P-Thr antibody.
(B) Phosphorylation of thylakoid proteins isolated form R- or FR-treated plants. Proteins were separated on 12% acrylamide gels and immunoblotted with P-Thr antibody.
(C) Analysis of L-LHCII subunits from GL-adapted thylakoids. Proteins were separated on 12% acrylamide gels and immunoblotted using antibodies against LHCB1 and LHCB2 and their phosphoforms (P). Right panel shows quantification of LHCB1, LHCB2, and their phosphorylated forms in nsi mutants (nsi-1 is marked with red circles and nsi-2 with blue triangles). Protein amounts were quantified from the blots and calculated as a percentage of the wild type from the respective replicate.
(D) Immunoblot analysis of L-LHCII docking site on PSI. Proteins were isolated from GL-adapted plants, separated on 12% acrylamide gels, and immunoblotted with PSAH and LHCA4 antibodies. The lower panels (Total) show blots after staining with REVERT total protein stain to verify equal loading; representative blots are shown from three biological replicates ([A] to [D]).
(E) Comparison of chloroplast protein complexes between the wild type and nsi in state 2. Upon conditions favoring plastoquinone pool reduction, L-LHCII trimers are phosphorylated, which results in the interaction of L-LHCII with PSI, mediated by PSAH (state 2 in the wild type). In contrast to the wild type, P-LHCII is not able to interact with PSI in nsi under state 2 conditions. The phenotype may result from defects in Lys acetylation of (1) PSAH and LHCII, which may hinder the PSI-LHCII interaction; (2) PSBP and LHCII, which may result in a strong interaction between PSII and L-LHCII; or (3) proteins involved in chloroplast ion homeostasis (PSBP and KEA1/KEA2), which may be required for the dynamic reorganization of thylakoid protein complexes.