Figures 1a/1b/1c.
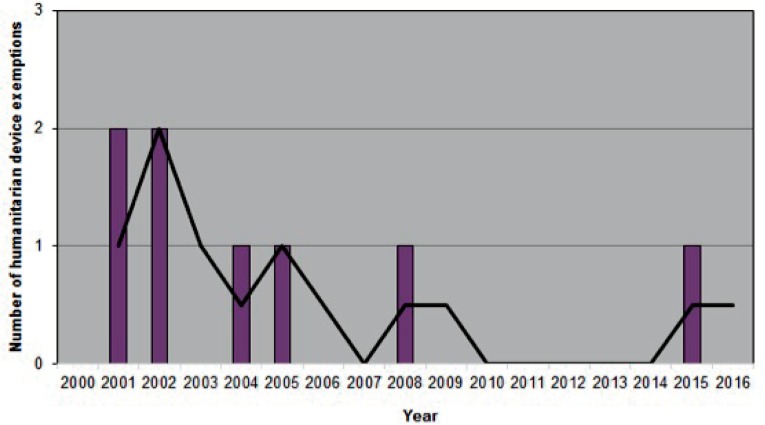
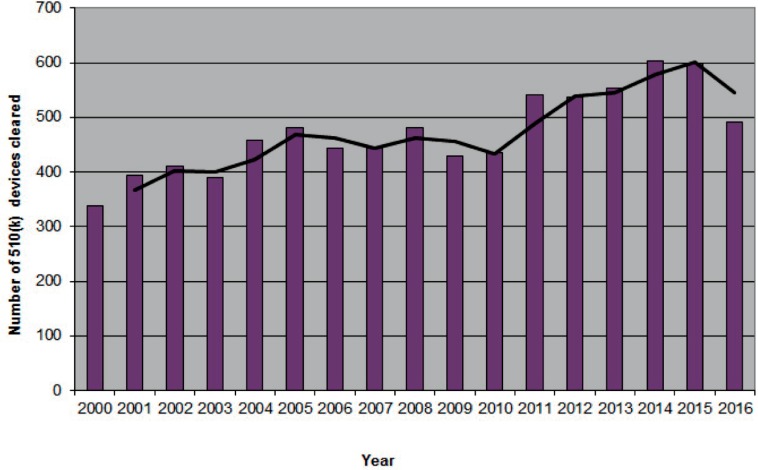
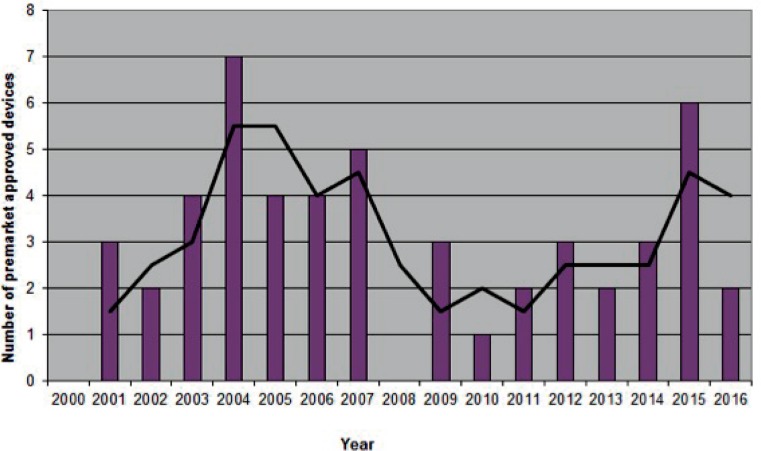
Number of orthopaedic devices advanced to market between 2000–2016
1a. Humanitarian device exemptions Data acquired from the Humanitarian Device Exemption Database found at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfHDE/hde.cfm. Advisory Committee: Orthopedic. Accessed February 27, 2017. Each device was counted only once.
1b. 510(k) clearances
Data acquired from the 510(k) Premarket Notification Database found at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm. Panel: Orthopedic. Accessed February 27, 2017.
1c. Premarket approvals
Data acquired from the Premarket Approval Database found at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm. Advisory Committee: Orthopedic. Supplement Type: Originals Only. Accessed February 27, 2017.