Abstract
Patients presenting with soft-tissue lumps and bumps are commonly encountered by both primary care physicians and orthopaedic surgeons. Though common, the initial evaluation and management of a soft-tissue mass can be challenging for many clinicians due to the considerable overlap in the presentation of benign and malignant tumors. Furthermore, a myriad of conditions, ranging from infection to trauma, can present with a soft-tissue mass. Subsequently, the correct diagnosis is often delayed or missed which may ultimately lead to inappropriate treatment. The propose of this article is to review the fundamental elements of a successful soft-tissue mass work-up and to provide the clinician with a systematic approach to the evaluation, diagnosis and management of the patient with a soft-tissue tumor.
Epidemiology
Soft-tissue tumors are common; however, defining the true incidence of these tumors is difficult given that most go unrecognized or simply do not present for medical evaluation when the individual is unconcerned with the presence of an asymptomatic mass. Most soft-tissue tumors are benign, outnumbering malignant tumors approximately 150:1 with roughly 20 malignant soft-tissue tumors per 1 million people in the United States.1 Although carcinomas can potentially metastasize to the soft-tissues and lymphomas may occasionally invade muscle plains, malignant or cancerous tumors of the soft-tissues are collectively referred to as sarcomas. Soft-tissue sarcomas can occur at any age, although the majority are seen in patients 40 years of age or older. When sarcomas occur in children, greater than 50% are Rhabdomyosarcomas. In young adults, synovial sarcoma and epithelioid sarcoma are the most commonly diagnosed. Sarcomas occur more frequently in the lower extremities than in the upper extremities by a 2:1 ratio.2 Nearly one-third of sarcomas occur in the trunk and pelvis and 10% occur in the head and neck.3
Presentation and Evaluation
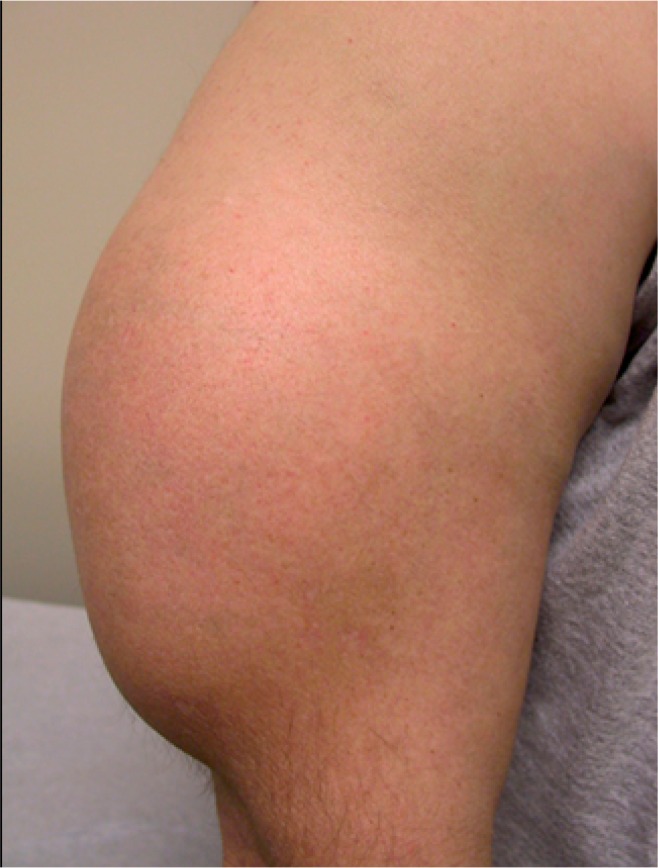
Patients with a soft-tissue tumor, whether benign or malignant, typically present with a painless, growing prominence. Subcutaneous tumors are often easily recognized, allowing the patient to isolate the exact location of the tumor and provide a detailed description of the nature and duration of their symptoms. In contrast, deep soft-tissue tumors located within the muscle or below the fascia may go unrecognized initially and are often not localized until the tumor has grown to a large size (> 10 cm). (Figure 1)4
Figure 1.
Benign soft-tissue mass of the arm.
(Murphy MD. Imaging of Soft Tissue Tumors. 2014.)
History
Despite the overlapping clinical features of benign and malignant soft-tissue tumors, in general, an accurate differential diagnosis can be formulated after a thorough history and physical exam has been performed. History taking should include questions designed to determine how long the mass has been present as well as the nature and duration of any associated symptoms. Any changes in the size or growth rate of the mass should be noted, as well as any prior history of cancer. Antecedent trauma should be noted as a history of trauma suggests the presence of a hematoma or possibly myositis ossificans. An abscess rises in the differential if the patient complains of constitutional symptoms in the setting of a slow healing wound or a rapidly enlarging, red, warm and tender mass. Crystalline or inflammatory arthropathy, including Gout or rheumatoid arthritis, can also produce a red, warm and tender soft-tissue tophus or nodule.
A common misconception is that patients with a malignant soft-tissue tumor will appear ill, as is often seen in patients with disseminated non-musculoskeletal malignancies. A lack of co-existent constitutional symptoms such as fever, chills, night sweats or unintentional weight loss, should not lower the clinician’s index of suspicion for malignancy. Such symptoms are rarely present, even in patients with metastatic disease.16 A slowly growing mass suggests a benign nature, whereas, rapid growth over a period of weeks to months is concerning for a malignancy. A mass with a “waxing and waning” size suggests the presence of a ganglion cyst or hemangioma, but it is not typically a characteristic of a sarcoma.
Many practitioners mistakenly believe that there is no need for concern unless a mass is painful, when in fact, most malignant lesions are asymptomatic early on. When symptoms do present, they are typically due to the size of the tumor causing mass effect on surrounding structures. When a benign tumor reaches a large size, it may result in the compression of vessels, nerves or vital organs. Thus, a benign tumor can be characterized as a “locally aggressive” tumor despite its inability to metastasize. In addition to localized pain, patients may experience neurogenic symptoms such as referred pain, paresthesia or a focal neurologic deficit if the mass compresses or incorporates a peripheral nerve.
Physical Exam
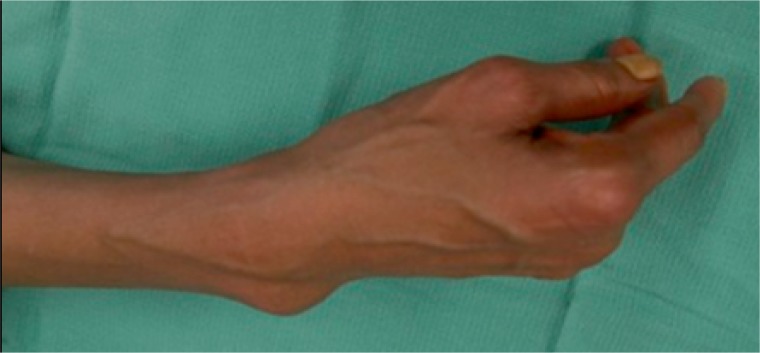
Distinguishing features that can aid in narrowing the diagnosis of a soft-tissue mass are the size, depth, consistency, and mobility of the mass. Typically, masses that are superficial and less than 5 cm in size tend to be benign, whereas, masses that are greater than 5 cm or found deep to the fascia have a higher likelihood of being malignant. However, one-third of sarcomas will present as a superficial mass.7 (Figure 2)4 A firm, fixed mass suggests a sarcoma or an underlying bony origin, although a desmoid tumor can present in this way as well. Benign masses are often soft and mobile, as is typical of a lipoma. In adults, most small superficial soft-tissue tumors are lipomas, whereas, in children most are hemangiomas.
Figure 2.
High-grade undifferentiated sarcoma in the wrist.
(Murphy MD. Imaging of Soft Tissue Tumors. 2014.)
Superficial bruising or ecchymosis is seen with a traumatic hematoma as it tracks along fascial planes. When bleeding within a tumor occurs, blood is typically contained within the tumor capsule and rarely reaches the superficial tissues. A warm, red, swollen or fluctuant mass is typical of an abscess; however, sarcomas can be warm to the touch secondary to tumor neovascularization and increased blood flow. Large tumors that compress the neurovascular structures can cause edema due to the dilation of peripheral veins or decreased pulses distal to a site of arterial compression. A loss of sensibility or motor function can occur in the distribution of a compressed peripheral nerve.
Some specific findings can narrow the differential diagnosis even further. Synovial or ganglion cysts will trans-illuminate with a pen light. Vascular lesions such as a hemangioma or arteriovenous malformation may have bruits or a palpable thrill. Peripheral nerve sheath tumors may have a positive Tinel’s sign or pain with compression. Ulceration of the overlying skin can be seen with epithelioid sarcoma. The clinician should carefully examine the skin and document the presence of any previous incision or scars. These findings can be critically important as they may represent the site of a prior biopsy or attempted surgical excision. Furthermore, the location of prior incisions becomes important for preoperative planning and may determine or alter the tumor surgeon’s surgical approach if an excision is required.
Diagnosis
Laboratory Studies
Currently, no specific laboratory studies exist that are of significant benefit in the evaluation of soft-tissue masses. Routine studies like a white blood count, erythrocyte sedimentation rate, and c-reactive protein may be elevated in a variety of conditions. Calcium and phosphorus levels may be abnormal in the setting of tumoral calcinosis. An elevated lactate dehydrogenase level is seen in patients with lymphoma. Uric acid levels can be elevated in gout. These tests are nonspecific and are generally of limited use.1
Imaging Studies
The evaluation and management of soft-tissue tumors often relies heavily on information gathered through imaging and may involve plain radiography, computed tomography (CT), ultrasound (US), and magnetic resonance imaging (MRI). Plain radiography, though it has a limited role in the definitive diagnosis and staging of soft-tissue tumors, should be obtained first to determine the presence or lack of bony involvement. Plain radiography may only reveal a non-specific alteration of normal soft-tissue shadowing. Though rare, bony involvement either by a benign or a malignant soft-tissue mass can be appreciated on plain radiographs and may be seen in the form of cortical erosion, cortical hyperostosis or a periosteal reaction.
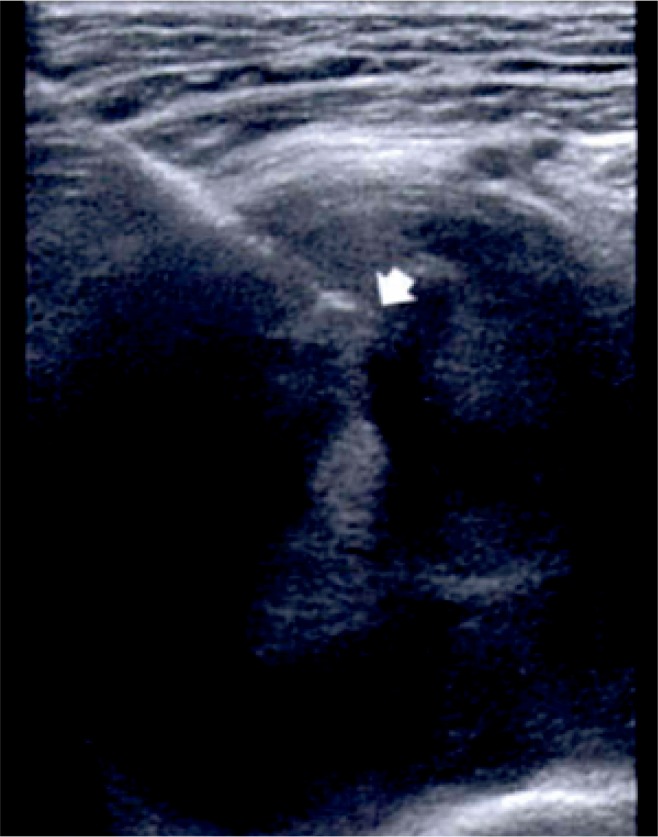
Ultrasound is another cost effective imaging modality with a high negative predictive value for soft tissue masses.8 US can help to determine the consistency (solid or cystic), relationship to adjacent structures, vascularity, size, and shape of the mass. The use of US may obviate the need for more costly imaging such as MRI, avoid unnecessary biopsy, and guide the appropriate treatment plan. However, when the diagnosis remains uncertain or concerning features of the mass are identified, further imaging should be performed. Importantly, US can be used intraoperatively for biopsy or excision and is especially valuable if the mass is not palpable.9 (Figure 3)4
Figure 3.
Ultrasound guided biopsy of a groin mass in a 56-year-old man. The tip of the core biopsy needle is seen (arrow) within the mass. Biopsy revealed schwannoma with central hemorrhage.
(Murphy MD. Imaging of Soft Tissue Tumors. 2014.)
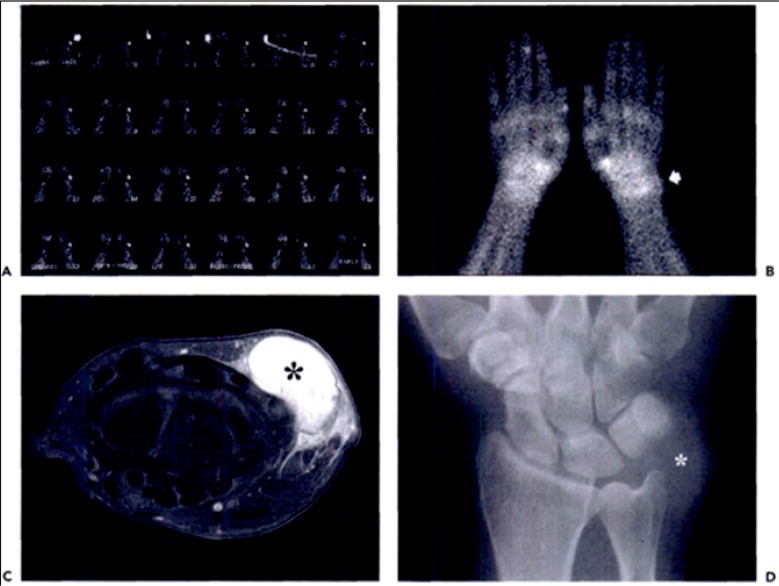
MRI is the most sensitive and specific imaging modality available in the evaluation of soft-tissue masses. MRI is the study of choice for the localization and staging of soft-tissue tumors as is offers the best delineation of the soft-tissue structures and the relationship of the mass with respect to neurovascular structures.1 10,11 T1-weighted MRI is best for defining anatomic relationships, while T2-weighted MRI identifies free extracellular water and tissue edema. Fat suppression techniques subtract the signal produced by adipose tissue and can better highlight abnormal fluid collections and areas of tumor transition. Gadolinium-enhanced MRI has become an important part of the workup for soft-tissue masses because it allows the clinician to differentiate between cystic and solid lesions. Cystic lesions will have rim enhancement, which is not present in solid lesions. This is especially important for differentiating between myxomas, myxoid liposarcomas, and fluid-filled cysts. (Figure 4)4
Figure 4.
High-grade undifferentiated sarcoma in the wrist. (A) Dynamic and (B) static (B) bone scintigraphy shows increased flow and tracer accumulation in the tumor (arrow). The corresponding contrast enhanced, fat-suppressed, axial T1 weighted MR image shows intense enhancement of the mass. (D) Anteroposterior radiograph of the wrist shows the mass (asterisk) with no adjacent osseous abnormality.
(Murphy MD. Imaging of Soft Tissue Tumors. 2014.)
The use of computed tomography (CT) for diagnosis of soft-tissue masses has declined as the role of MRI has evolved. CT is often the imaging modality of choice for patients who cannot undergo MRI, including those with pacemakers or indwelling cardiac stents. Unlike MRI, which is limited in the setting of mineralization secondary to the variable signal intensity of calcium, CT is better able to delineate any bony involvement and distinguish calcification from ossification. 12
Positron emission tomography (PET) is commonly used in conjunction with CT (PET-CT) and provides precise anatomic correlation of tumors and information about metabolic activity within a soft-tissue tumor. PET-CT detects positron emission decay from an administered radioisotope and generates an image of the entire body. The amount of radiotracer activity within the cells reflects metabolic activity. Typically, high-grade malignant soft-tissue tumors have high rates of glucose metabolic activity. Although PET-CT is not used for initial evaluation of soft-tissue sarcoma, it has been found to be useful to assess treatment response after systemic therapy or to identify recurrence. 13
Biopsy
Though it is a seemingly simple procedure, biopsy of soft-tissue masses is technically challenging and potentially fraught with complications. It is recommended that the biopsy of soft-tissue masses be deferred to a tumor-trained orthopaedic surgeon when possible and is preferably performed by the same surgeon who will perform the definitive surgery if indicated. 14 Careful planning is required to determine the exact location and approach to the mass. When possible, the surgeon should consult with both the radiologist and pathologist prior to proceeding to biopsy.
Two forms of soft-tissue biopsy exist: needle biopsy and open biopsy. Needle biopsies involve the passing of a needle into a mass for tissue sampling. When possible, the needle tract should pass through the muscle that is in contact with the tumor and away from major blood vessels or nerves. The tract must also be placed in a location that will allow for the tissue to be excised at the time of definitive surgery.
Needle biopsy is further divided into fine-needle aspiration (FNA) and core needle biopsy (CNB). FNA involves aspirating the mass with a 20-gauge needle or smaller, whereas, CNB uses a larger bore needle and requires multiple needle passes to gather tissue. The advantages of FNA include that it is relatively inexpensive, can be done in the clinic with minimal local anesthetic, results in limited soft-tissue contamination, and can be processed in minutes if a pathologist is available. FNA has a wide range of reported accuracy, especially in diagnosing sarcoma with sensitivity of 86–100%, specificity of 36–100%, and diagnostic accuracy of 21.9 – 98%. However, FNA yields only tumor cells which can be used for cytology studies, as opposed to CNB which provides ample tissue for additional testing. CNB allows for the cell structure and cellular matrix to be examined by pathology, improving diagnostic accuracy. CNB has a reported sensitivity of 81.8–100%, specificity of 91–100%, and diagnostic accuracy of 72.7–100%.15 While needle biopsies have the potential to be a cost-effective method for the diagnosis of soft-tissue tumors, the accuracy of tumor grade and histology subtype varies and is dependent on the pathologist’s experience. 16–18
In contrast to needle biopsies, open biopsy is an even more technically demanding procedure. Open biopsy can also be subdivided into two types: incisional and excisional biopsy. In an incisional biopsy, a portion of the tumor is removed to make a diagnosis. Excisional biopsy implies a planned marginal resection of the mass and is reserved for masses that are thought to be benign, such as a lipoma or peripheral nerve sheath tumor. Open biopsies yield a larger tissue sample which allows for molecular-diagnostic testing. The smallest incision possible (less than 5 cm) should be used to allow for an adequate sample with the least risk of complication. Incisions should be longitudinal and placed along the future limb salvage incision, or just parallel to it. Any tissue exposed during open biopsy must be removed during definitive surgery, including blood vessels and nerves. Poorly placed biopsy sites may adversely affect the surgeon’s ability to resect the tumor, significantly increasing recurrence and need for further treatment that may include amputation.15 16–18
Management
Resection
There are 4 types of resection procedures that can be performed: intra-lesional, marginal, wide, and radical. Figure 5 illustrates the surgical resection types described by Enneking and Dunham. A key principle of tumor surgery is to attempt to avoid inter-nervous plans or the violation of multiple compartments. Approaches that traverse muscle bellies and limit contamination are selected. Intra-lesional resection involves incising the tumor capsule and removing only a part of the mass. This is preferred for benign masses such as a hematoma. Marginal excision refers to removal of the mass in its entirety while keeping the reactive zone or thin capsule intact. This is preferred for benign tumors such as lipomas, giant cell tumors of tendon sheath or neurofibromas. Wide excision involves removing the tumor as well as a cuff of normal tissue surrounding the mass. This is the preferred treatment for locally aggressive tumors and sarcomas. Finally, radical resection refers to removal of the entire compartment, which is rarely required.19,20
Adjuvant Therapies
Currently available adjuvant therapies fall into 3 categories: local treatment (cryotherapy, radiofrequency ablation (RFA) etc.), chemotherapy, and radiation therapy. Cryotherapy and RFA have been utilized effectively in the management of metastatic lesions or for tumor recurrence where surgical resection is not possible. Both have successfully been performed to manage soft tissue sarcomas, desmoid tumors, and metastatic diseases.21
External beam radiation is another modality that can be used for local tumor control. Radiation works by generating free radicals which then inhibit tumor cell DNA synthesis, ultimately leading to apoptosis. Thus, radiation primarily effects tumor cells with high turnover rate or mitotic index. However, rapidly dividing skin cells and hematopoietic cells are also affected. 23 Therefore, radiation is a useful adjunct in the treatment of those tumors known to have a high mitotic index such as Ewing’s sarcoma, lymphoma, metastatic disease, and soft tissue sarcomas. Radiation therapy can be utilized pre-operatively to shrink the size of the tumor, postoperatively to treat unresected margins or unseen satellite lesions, or even as palliative therapy in lesions that are not amenable to complete resection. In one report, resection surgery alone led to a recurrence in 22 of 64 patients (34%) as compared to 11 of 69 (16%) when surgery was accompanied by either pre- or post-operative radiation therapy.20
Chemotherapy is an adjunct that acts systemically. While local adjuvants, radiation and surgery treat local disease, chemotherapies aim to eliminate distant or disseminated tumor cells. The goal of chemotherapy can be curative or palliative. Commonly used chemotherapeutic agents include doxorubicin which acts by binding tumor cell DNA and inhibits the S phase of the cell cycle, as well as cisplatin and cyclophosphamide which inhibit the tumor cell G2 phase DNA crosslinking and preventing cell division. Methotrexate is another commonly used agent which blocks folate synthesis leading to accumulation of a toxic inhibitory substrate. These and other agents may be used alone or in combination to treat systemic disease caused by a malignant soft tissue sarcoma. However, as it is with radiation therapy, the rapidly dividing cells of healthy tissues are also affected leading to common side effects which may include easy bleeding, anemia, thrombocytopenia, hair loss, gastrointestinal discomfort, nausea and vomiting. 20,24,25
Unplanned Oncologic Excision
A major concern of the orthopaedic and oncologic community is the incidence of unplanned resection. Reports have shown that 5–80% of patients with soft tissue sarcoma undergo unplanned resection, leading higher local recurrence and increased morbidity and mortality. 26 If the diagnosis is in question at the time of excision, a frozen section should be obtained to evaluate for malignancy. Additionally, the surgeon should take care to ensure that an adequate sample is obtained to establish a diagnosis. Conversely, overzealous dissection or exposure should be avoided as this could lead to further contamination.
Summary
The successful evaluation and management of soft tissues masses requires a systematic approach. A thorough history and physical examination in conjunction with appropriate imaging studies are essential for an accurate diagnosis. If the diagnosis remains unclear, biopsy should be performed by a musculoskeletal oncologist who will ultimately be responsible for the definitive surgery. The surgical resection of a soft-tissue tumor demands strict adherence to oncological surgical principles and techniques, mainly the avoidance of health tissue plane violation. Adjuvant therapies such as RFA, cryotherapy, radiation therapy, and systemic chemotherapy provided to the patient utilizing a multidisciplinary team approach will improve patient care. Adherence to these practices will reduce the incidence of unplanned tumor resection and lead more successful outcomes in the treatment of soft-tissue sarcomas.
Biography
Dane J. Church, MD, is a Chief Resident, and John Krumme, MD, MSMA member since 2010, is a Senior Resident in the Department of Orthopaedic Surgery at the University of Missouri - Kansas City School of Medicine. Suhel Kotwal, MD, (above), is Assistant Professor in the Department of Orthopaedic Surgery at UMKC School of Medicine.
Contact: Suhel.kotwal@tmcmed.org

Footnotes
Disclosure
None reported.
References
- 1.Mayerson JL, Scharschmidt TJ, Lewis VO, Morris CD. Diagnosis and Management of Soft-tissue Masses. J Am Acad Orthop Surg. 2014;22(11):742–750. doi: 10.5435/JAAOS-22-11-742. [DOI] [PubMed] [Google Scholar]
- 2.Tukiainen E, Böhling T, Huuhtanen R. Soft tissue sarcoma of the trunk and extremities. Scand J Surg. 2003;92(4):257–263. doi: 10.1177/145749690309200404. [DOI] [PubMed] [Google Scholar]
- 3.Wibmer C, Leithner A, Zielonke N, Sperl M, Windhager R. Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann Oncol. 2010;21(5):1106–1111. doi: 10.1093/annonc/mdp415. [DOI] [PubMed] [Google Scholar]
- 4.Kransdorf Mark J, Murphy MD. Imaging of Soft Tissue Tumors. Philadelphia: 2014. [Google Scholar]
- 5.Sim FH, Frassica FJ, Frassica DA. Soft-Tissue Tumors: Diagnosis, Evaluation, and Management. J Am Acad Orthop Surg. 1994:26–33. doi: 10.1079/SUM200196. (Dc): [DOI] [PubMed] [Google Scholar]
- 6.Rydholm A, Gustafson P, Rööser B, Willén H, Berg NO. Subcutaneous sarcoma. A population-based study of 129 patients. J Bone Joint Surg Br. 1991;73(4):662–667. doi: 10.1302/0301-620X.73B4.2071656. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=2071656&retmode=ref&cmd=prlinks%5Cnpapers2://publication/uuid/63B63D24-1397-4833-BCC7-ECA55460D2F5. [DOI] [PubMed] [Google Scholar]
- 7.Smith SE, Salanitri J, Lisle D. Ultrasound evaluation of soft tissue masses and fluid collections. Semin Musculoskelet Radiol. 2007;11(2):174–191. doi: 10.1055/s-2007-1001882. [DOI] [PubMed] [Google Scholar]
- 8.Farfalli GL, Aponte-Tinao La, Rasumoff A, Ayerza Ma, Muscolo DL. Intraoperative ultrasound assistance for excision of impalpable musculoskeletal soft tissue tumors. Orthopedics. 2011;34(9):e570–3. doi: 10.3928/01477447-20110714-03. [DOI] [PubMed] [Google Scholar]
- 9.Papp DF, Khanna aJ, McCarthy EF, Carrino Ja, Farber AJ, Frassica FJ. Magnetic resonance imaging of soft-tissue tumors: determinate and indeterminate lesions. J Bone Joint Surg Am. 2007;89(Suppl 3):103–115. doi: 10.2106/JBJS.G.00711. [DOI] [PubMed] [Google Scholar]
- 10.Ostlere S, Graham R. Imaging of soft tissue masses. Imaging. 2005;17(3):268–284. doi: 10.1259/imaging/74338804. [DOI] [Google Scholar]
- 11.Subhawong TK, Fishman EK, Swart JE, Carrino JA, Attar S, Fayad LM. Soft-tissue masses and masslike conditions: What does CT add to diagnosis and management? Am J Roentgenol. 2010;194(6):1559–1567. doi: 10.2214/AJR.09.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzeng CWD, Smith JK, Heslin MJ. Soft Tissue Sarcoma: Preoperative and Postoperative Imaging for Staging. Surg Oncol Clin N Am. 2007;16(2):389–402. doi: 10.1016/j.soc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Mankin HJF, Mankin CJLST, Simon MA. The Hazards of the Biopsy, Revisited FOR THE MEMBERS OF THE MUSCULOSKELETAL TUMOR SOCIETY*. J bone Jt Surg. 1996;78-A(5):656–663. doi: 10.1097/01241398-199611000-00060. [DOI] [PubMed] [Google Scholar]
- 14.Kasraeian S, Allison DC, Ahlmann ER, Fedenko AN, Menendez LR. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin Orthop Relat Res. 2010;468(11):2992–3002. doi: 10.1007/s11999-010-1401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pohlig F, Kirchhoff C, Lenze U, et al. Percutaneous core needle biopsy versus open biopsy in diagnostics of bone and soft tissue sarcoma: a retrospective study. Eur J Med Res. 2012;17(1):29. doi: 10.1186/2047-783X-17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh HK, Kilpatrick SE, Silverman JF. Fine needle aspiration biopsy of soft tissue sarcomas: utility and diagnostic challenges. Adv Anat Pathol. 2004;11(1):24–37. doi: 10.1097/00125480-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Liu PT, Valadez SD, Chivers FS, Roberts CC, Beauchamp CP. Anatomically based guidelines for core needle biopsy of bone tumors: implications for limb-sparing surgery. Radiographics. 2007;27(1):189–205. doi: 10.1148/rg.271065092. discussion 206. [DOI] [PubMed] [Google Scholar]
- 18.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60(6):731–746. [PubMed] [Google Scholar]
- 19.Mendenhall WM, Indelicato DJ, Scarborough MT, et al. The management of adult soft tissue sarcomas. Am J Clin Oncol. 2009;32(4):436–442. doi: 10.1097/COC.0b013e318173a54f. [DOI] [PubMed] [Google Scholar]
- 20.Cornelis F, Havez M, Lippa N, et al. Radiologically guided percutaneous cryotherapy for soft tissue tumours: A promising treatment. Diagn Interv Imaging. 2013;94(4):364–370. doi: 10.1016/j.diii.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Belfiore MD, MP Ronza, MD FM, Della Volpe T, Pascale M, Belfiore G. Long-term local disease control in a recurrent soft-tissue sarcoma of the thigh treated by radiofrequency ablation. J Surg Oncol. 2015;111(6):708–710. doi: 10.1002/jso.23877. [DOI] [PubMed] [Google Scholar]
- 22.External Beam Radiation Therapy. 2002;23(4):225–237. doi: 10.1007/978-1-4939-1145-5. 09 C. [DOI] [Google Scholar]
- 23.Machak GN, Tkachev SI, Solovyev YN, et al. Neoadjuvant chemotherapy and local radiotherapy for high-grade osteosarcoma of the extremities. Mayo Clin Proc. 2003;78(2):147–155. doi: 10.4065/78.2.147. [DOI] [PubMed] [Google Scholar]
- 24.Walczak BE, Irwin RB. Sarcoma Chemotherapy. J Am Acad Orthop Surg. 2013;21:480–491. doi: 10.5435/JAAOS-21-08-480. [DOI] [PubMed] [Google Scholar]
- 25.Tedesco NS, Henshaw RM. Unplanned Resection of Sarcoma. J Am Acad Orthop Surg. 2016;24(3):150–159. doi: 10.5435/JAAOS-D-15-00074. [DOI] [PubMed] [Google Scholar]