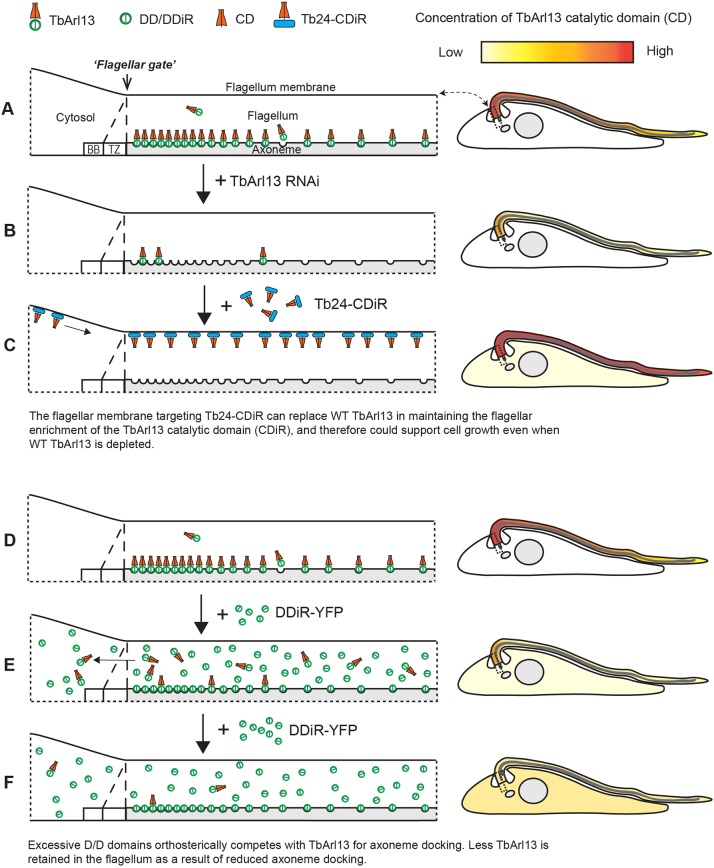
Fig. 7.
A diffusion-retention model accounts for flagellar enrichment of TbArl13, which is critical for TbArl13 cellular functions. (A,D) In wild-type cells, TbArl13 is specifically enriched in the flagellum by means of axoneme docking through the D/D domain (Figs 1 and 2), possibly via its interaction with a putative axonemal docking partner. BB, basal body; TZ, transition zone. (B) TbArl13-RNAi is lethal for the cell (Fig. 3). (C) Expression of Tb24–CDiR–YFP could rescue cell growth in the absence of endogenous TbArl13 (Fig. 4), because the flagellar enrichment of the catalytic domain of TbArl13 is restored through Tb24 (Fig. 4 and Fig. S6D). (E,F) As the D/D domain is sufficient for axonemal docking, excess amounts of DDiR–YFP compete with native TbArl13 for limited docking sites (Fig. 2). As more TbArl13 molecules diffuse out of the flagellum, overall flagellar enrichment of TbArl13 is compromised and results in a dominant-negative effect.