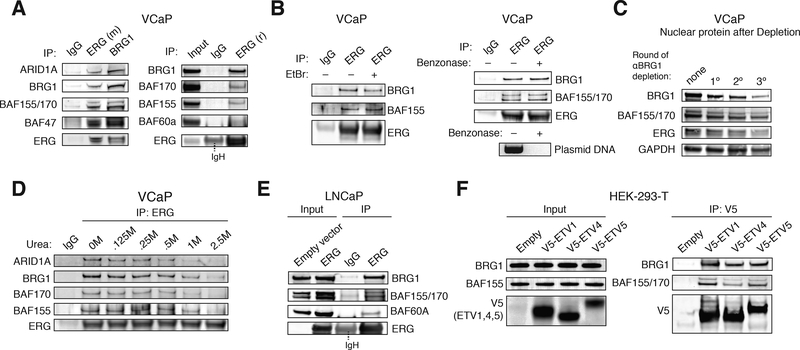
Figure 2.
In-solution binding of ERG to BAF complexes in prostate cancer.
(A) Immunoprecipitation using anti-ERG (mouse monoclonal) and anti-BRG1 antibodies (left); IP with an alternate anti-ERG (rabbit polyclonal) antibody (right), in VCaP cell nuclear extracts.
(B) Immunoprecipitation using IgG and anti-ERG antibodies, with or without ethidium bromide (EtBr) treatment (left). Immunoprecipitation using IgG and anti-ERG antibodies with or without treatment of benzonase, presence of plasmid DNA indicated for benzonase treatment control (right).
(C) Immunodepletion studies performed on VCaP cell nuclear extracts using an anti-BRG1 antibody.
(D) Urea denaturation analysis performed on anti-ERG IPs from VCaP nuclear extracts treated with [urea]= 0–2.5M.
(E) Nuclear protein input and anti-ERG IP on nuclear extracts from LNCaP cells in empty and ERG conditions.
(F) Input (left) and anti-V5 IP (right) in HEK-293-T cells transfected with V5-ETV1, V5-ETV4, V5-ETV5 or empty vector.