TABLE 1.
Evidence summary on the effectiveness of probiotic therapy in modulation of gastrointestinal dysfunction in children with ASD1
| Study | |||||
|---|---|---|---|---|---|
| Shaaban et al. (2017) (37) | Tomova et al. (2015) (29) | West (2013) (38) | Kaluzna-Czaplinska and Blaszczyk (2012) (31) | Parracho et al. (2010) (36) | |
| Study design | Prospective, open-label noncontrolled trial | Noncontrolled trial | Noncontrolled trial | Noncontrolled trial | Double-blind, placebo-controlled, crossover trial |
| Location | Egypt | Slovakia | USA | Poland | United Kingdom |
| Participants, n | 60 Children: 30 ASD, 30 age-/sex-matched controls (relatives) | 29 Children: 10 ASD, 9 non-ASD siblings, 10 non-ASD controls | 33 Children with ASD | 22 Children with ASD | 22 Children with ASD |
| Age | 5–9 y (mean age: 84.77 ± 16.37 mo) | ASD = 2–9 y: non-ASD siblings = 5–17 y; non-ASD controls = 2–11 y | 3–16 y (mean age = 7.92 y) | 4–10 y (mean age: 5.6 ± 1.6 y) | Males = 9.2 ± 2.4 y; females = 8.5 ± 2.1 y (5 withdrew before second feeding arm) |
| Sex | 19 boys (63.3%); 11 girls (36.7%) | ASD = 90% males; non-ASD siblings = 77.7% males; non-ASD controls = 100% males | Not reported | 91% males | 91% males |
| Diagnosis/classification of participants | ASD (DSM-V) | ASD (ICD-10 criteria); non-ASD siblings; non-ASD controls | ASD (no reported criteria for ASD diagnosis) | ASD (DSM-IV criteria) | ASD (no reported criteria for ASD diagnosis) |
| Strain of probiotic | 3 strains: Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacteria longum | “Children Dophilus”: Blend of: 3 strains of Lactobacillus (60%), 2 strains of Bifidumbacteria (25%), 1 strain of Streptococcus (15%) (exact strain information not provided) | Delpro capsule: 2 billion CFUs of each of the following: Lactobacillus delbrueckii, L. acidophilus, Lactobacillus casei, B. longum, Bifidobacteria bifidum; with an additional 8 mg Del-immune V powder | L. acidophilus | Lactobacillus plantarum WCFS1 |
| Dose of probiotic | 5 g of powder/d (each gram contained 100 × 106 CFUs of each strain) | Not provided | 10 billion CFUs total | 5 × 109 CFUs | 4.5 × 1010 CFUs |
| Duration of probiotic therapy | 1 time/d for 3 mo | 3 times/d for 4 mo | 1 capsule, 3 times/d for 21 d | 2 times/d for 2 mo | Daily for 3 wk |
| Compliance | Not reported, but parents were contacted weekly regarding compliance | Not measured | Not measured; also instructed to discontinue any other concomitant probiotics | Not measured | Not measured |
| GI symptoms at baseline | GI symptoms assessed include constipation, diarrhea, stool consistency and smell, flatulence, and abdominal pain | Parent questionnaire at baseline only | 84% had moderate or severe constipation at baseline (based on ATEC); 56% had moderate or severe diarrhea at baseline (based on ATEC) | No objective reporting provided; authors indicated all children had “severe” GI problems (abdominal pain, constipation, diarrhea) | Parents recorded GI function and symptoms in a diary throughout the study |
| Measurement of GI symptoms | 6-GSI used to assess GI symptoms (lower score = fewer GI symptoms) | Parent questionnaire at baseline only | 21-d stool frequency diary before and after intervention; fourth domain in ATEC includes 2 questions measuring severity of diarrhea and constipation | No | Parents recorded GI function and symptoms in a diary throughout the study |
| Measurement of gut microbiota | Yes—pre- and post-treatment fecal specimens were collected to assess fecal microbial composition | Yes—pre- and post-treatment fecal specimens were collected to detect gut microflora and cytokine levels | No | Measured pre- and post- treatment urinary DA, which is a metabolite of Candida, and DA-to-LA ratio, which is a marker of invasive candidiasis; fecal specimens were not studied | Yes—fecal specimens were used to detect gut microflora; collected before and after each feeding arm and the end of each washout period |
| Description of baseline gut microflora and comparison to control | Yes—baseline fecal microflora was assessed for presence of Lactobacilli and Bifidobacteria | Yes—baseline gut microflora for participants with and without ASD was described | No | Described baseline and post–probiotic DA and DA-to-LA ratio levels | Yes—baseline, feeding arm, and washout bacterial population levels were described; unable to compare to individuals without ASD because they were not included in this study |
| Food intake | Not recorded | Not recorded | Not recorded | 45% of participants had a “restricted diet” and the authors noted that all of the children were on a “sugar-free diet”; no further clarification provided | Not recorded |
| Additional psychological testing | ADOS/ADI-R | CARS, ADI | None | None | DBC-P/TBPS |
| Behavior analysis | ATEC was administered pre- and postintervention | CARS and ADI administered only at baseline | ATEC was administered before and after Delpro intervention; higher ATEC scores suggest greater severity of ASD symptoms | None | Yes—change in behavior with placebo and probiotic treatment was evaluated using DBC-P tool and TBPS score |
| Blood samples or additional samples/measurements | 6-GSI; anthropometric measurements | Plasma—oxytocin, testosterone and dehydroepiandrosterone-sulfate concentrations; fecal—TNF-α | No | No | No |
| Attrition | Not reported | Not reported | 75.7% for ATEC scores; 63.6% for stool diaries | Not reported | 27.4% |
| Results | Children with ASD had lower Bifidobacteria levels than did age- and sex-matched controls at baseline and fecal Lactobacilli and Bifidobacteria concentrations increased as a result of supplementation (P < 0.0001) | Children with ASD and their siblings had more GI dysfunction than did controls (P < 0.05) with GI symptom severity and autism severity being strongly, positively correlated (R = 0.78, P = 0.01) | 52% of the total respondents reported severe constipation at baseline with a decline to 20% reporting severe constipation after treatment; 20% of the total respondents reported severe diarrhea at baseline with a decline to none after treatment | Urine DA (a metabolite of Candida species) level was higher in children before probiotic supplementation (160.04 ± 22.88 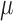 mol/mmol creatinine; P < 0.05) mol/mmol creatinine; P < 0.05) |
Probiotic therapy significantly increased the amount of Lactobacilli/Enterococci (P < 0.05) and decreased the Clostridium coccoides (P < 0.05) found in the stool samples of children with ASD as compared with placebo |
| 6-GSI scores increased/improved (P < 0.0001) in children with ASD after 3 mo of probiotic supplementation, specifically in the symptoms of constipation (P < 0.01), abdominal pain (P < 0.002), flatulence (P < 0.037), and stool consistency (P < 0.023); reduced constipation scores were significantly negatively correlated with increased fecal Bifidobacteria (r = –0.441, P < 0.015) | Bacteroidetes-to-Firmicutes ratio was lower (P < 0.05) and Lactobacillus was higher (P < 0.05) in children with ASD as compared with controls | Of the 25 ATEC responders, there was a significant decrease across all ATEC domain scores after finishing 21 d of Delpro intervention (P < 0.05 for all domains: speech/language/communication, sociability, sensory/cognitive awareness, health/physical/behavior). Mean ATEC scores decreased from 72.8 before treatment to 58.3 after treatment (P < 0.05) | Urine DA-to-LA ratio (a marker of invasive candidiasis) decreased from a mean of 3.15 ± 0.41 before probiotic treatment to a mean of 2.77 ± 0.28 after probiotic treatment | 94% (n = 16) of the diaries recording bowel function and GI symptoms during the feeding arms of the trial were returned while only 17% (n = 3) of the diaries recording the same symptoms during the washout period were returned | |
| ATEC scores significantly improved after 3 mo of probiotic supplementation (P < 0.0001), specifically, speech/language/communications subscale (P < 0.017), health/physical/behavioral (P < 0.0001), sensory/cognitive awareness (P < 0.026), and sociability (P < 0.001) all improved after supplementation; GI symptom improvement (as measured by 6-GSI) was strongly correlated to improved autism severity (as measured by ATEC) (r = 0.674, P = 0.0001) | Children with ASD had higher, but not significant, amounts of Clostridia cluster 1 and Desulfovibrio than controls or siblings | Of those who experienced a decrease in the fourth domain after Delpro intervention, 57% and 52% reported a decrease in constipation and diarrhea, respectively. One participate reported an increase in constipation and 2 reported an increase in diarrhea | There was no difference in the number of daily bowel movements noted between the probiotic or placebo arms | ||
| In children with greater severity of GI symptoms, there were lower amounts of Clostridia cluster 1 and Desulfovibrio and a lower Bacteroidetes-to-Firmicutes ratio than in those children with mild GI symptoms, albeit not significantly. Conversely, children with more severe autism (CARS ≥50) had greater Clostridia cluster 1 and Desulfovibrio and a lower Bacteroidetes-to-Firmicutes ratio than in those children with mild autism (not significant) | Of the 21 pre- and 18 post-stool log responders, stool frequency increased by 0.2 d, which was not significant | There were significantly fewer “hard” stools reported during the probiotic feeding arm (8.1%) vs. placebo (15.9%) (P < 0.01) | |||
| There was a very strong correlation between Desulfovibrio and the ADI restricted/repetitive behavior score (R = 0.83, P < 0.05) | Stools were significantly more “formed” during the probiotic feeding arm (73.3%) vs. placebo (64.8%) (P < 0.01) | ||||
| Probiotic therapy elicited a decrease in Firmicutes, which increased the Bacteroidetes-to-Firmicutes ratio to levels seen in the children without ASD. A similar effect was seen with regard to decreasing Bifidobacterium in children with ASD to levels found in controls | No significant difference noted for abdominal pain, bloating, or flatulence between either treatment | ||||
| Fecal TNF-α concentrations were higher in children with ASD and their siblings compared with children without ASD, although not significantly, and there was a strong correlation between TNF-α concentrations and GI symptoms (R = 0.78, P < 0.05). There was also a trend toward a correlation between TNF-α concentrations and severity of ASD, as measured by ADI (R = 0.7, P = 0.06). Probiotic therapy significantly decreased TNF-α concentrations (2-tailed P < 0.05) | 88% (n = 15) of TBPSs were completed with no significant differences noted between the treatments; however, baseline scores were significantly higher (P < 0.05) than scores measured during the probiotic and placebo periods | ||||
| Limitations | Children with ASD with dual diagnoses or with GI disorders were not included (concurrent neurodevelopmental or psychiatric conditions were excluded) | GI measurement was conducted at baseline only with parent questionnaire, which would have introduced bias. | Unclear if similar results would be found with just probiotic supplementation (without immune modulator) | Objective measurement of GI symptoms was not collected pre- or postprobiotic supplementation | The probe set used in this study obtained <50% coverage of the total bacterial count |
| This was a small, nonblinded, convenience sample, and there was no control for diet or additional therapy, even though the authors noted that the children in this study were receiving ongoing behavioral therapy | Behavior analysis was only done at baseline; therefore, we are unable to determine if probiotics influenced alteration in ASD symptoms | Potential risk of bias due to industry affiliation | Change in ASD symptoms were reported; however, no description of how behaviors were measured pre- and postprobiotic supplementation | Three adverse events were reported and included a 3-d skin rash after starting the probiotic, diarrhea during the probiotic feeding arm, and weight loss during the probiotic feeding arm of the trial | |
| No control for diet, medication, or additional therapy | Convenience sample without control group or control of confounding variables; group may be skewed as heavily represented by those with severe constipation or diarrhea | Design of the trial may have been too ambitious for this population; the significant drop-out rate affected the statistical power of the trial | |||
| No analysis of change in gut microbiome or intestinal permeability after treatment | High interindividual variability found in this trial suggests that future research may need to focus the intervention in a more defined subgroup of ASD (e.g., similar baseline fecal microbiota or similar GI symptoms) to elucidate more meaningful results | ||||
| Compliance not measured and duration of treatment may not be ideal | Lack of change in behavior may have been hindered by the short-term treatment period. Exploration of change in behavior using a single-subject design may be warranted | ||||
| A more objective tool for measuring GI symptoms and severity may have improved reporting | |||||
| Quality of evidence (27) | Neutral | Neutral | Negative | Negative | Neutral |
ADI, Autism Diagnostic Interview; ADI-R, Autism Diagnostic Interview – Revised; ADOS, Autism Diagnostic Observational Schedule; ASD, autism spectrum disorder; ATEC, Autism Treatment Evaluation Checklist; CARS, Childhood Autism Rating Scale; DA, d-arabinitol; DBC-P, Development Behavior Checklist—Primary Carer Version; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM-V, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; GI, gastrointestinal; ICD-10, International Classification of Diseases, 10th Revision; LA, l-arabinitol; TBPS, Total Behavior Problem Score; 6-GSI, modified GI Severity Index.
