Figure 1.
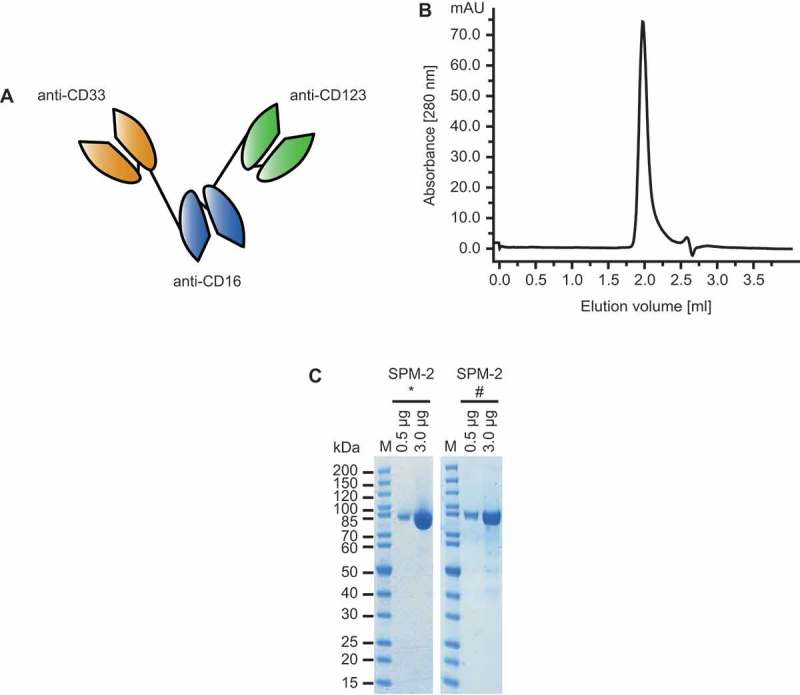
Design and properties of purified SPM-2. A. Molecular structure of triplebody SPM-2 (schematic). Single chain Fragment variable (scFv) domains, consisting of one VH domain joined to one VL domain, are connected into a single polypeptide chain. Black lines: flexible (Gly4Ser) linkers between the domains. One of the two distal scFv domains (orange) is specific for CD33 and the other (green) for CD123. The central scFv domain (blue) binds to CD16 and permits recruitment of various CD16-bearing types of effector cells, including NK and gamma delta T cells. B. After capture with a metal-ion affinity reagent and chromatographic purification the protein appeared as monomeric peak by size exclusion chromatography. C. SMP-2 was analyzed by SDS-PAGE directly after purification (left) and after storage for 12 months at 4°C (right). Only very small quantities of degradation products were observed. *: protein analyzed directly after purification, #: protein analyzed after 12 months of storage at 4°C; M: molecular weight marker in kDa.
