Abstract
Background and objectives
Older patients with ESKD experience rapid declines in executive function after initiating hemodialysis; these impairments might lead to high rates of dementia and Alzheimer’s disease in this population. We estimated incidence, risk factors, and sequelae of diagnosis with dementia and Alzheimer’s disease among older patients with ESKD initiating hemodialysis.
Design, setting, participants, & measurements
We studied 356,668 older (age ≥66 years old) patients on hemodialysis (January 1, 2001 to December 31, 2013) from national registry data (US Renal Data System) linked to Medicare. We estimated the risk (cumulative incidence) of diagnosis of dementia and Alzheimer’s disease and studied factors associated with these disorders using competing risks models to account for death, change in dialysis modality, and kidney transplant. We estimated the risk of subsequent mortality using Cox proportional hazards models.
Results
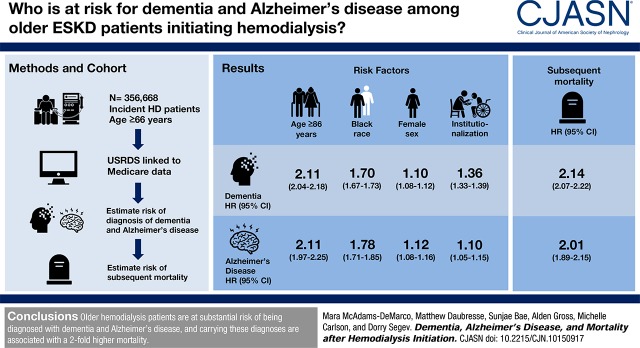
The 1- and 5-year risks of diagnosed dementia accounting for competing risks were 4.6% and 16% for women, respectively, and 3.7% and 13% for men, respectively. The corresponding Alzheimer’s disease diagnosis risks were 0.6% and 2.6% for women, respectively, and 0.4% and 2.0% for men, respectively. The strongest independent risk factors for diagnosis of dementia and Alzheimer’s disease were age ≥86 years old (dementia: hazard ratio, 2.11; 95% confidence interval, 2.04 to 2.18; Alzheimer’s disease: hazard ratio, 2.11; 95% confidence interval, 1.97 to 2.25), black race (dementia: hazard ratio, 1.70; 95% confidence interval, 1.67 to 1.73; Alzheimer’s disease: hazard ratio, 1.78; 95% confidence interval, 1.71 to 1.85), women (dementia: hazard ratio, 1.10; 95% confidence interval, 1.08 to 1.12; Alzheimer’s disease: hazard ratio, 1.12; 95% confidence interval, 1.08 to 1.16), and institutionalization (dementia: hazard ratio, 1.36; 95% confidence interval, 1.33 to 1.39; Alzheimer’s disease: hazard ratio, 1.10; 95% confidence interval, 1.05 to 1.15). Older patients on hemodialysis with a diagnosis of dementia were at 2.14-fold (95% confidence interval, 2.07 to 2.22) higher risk of subsequent mortality; those with a diagnosis of Alzheimer’s disease were at 2.01-fold (95% confidence interval, 1.89 to 2.15) higher mortality risk.
Conclusions
Older patients on hemodialysis are at substantial risk of diagnosis with dementia and Alzheimer’s disease, and carrying these diagnoses is associated with a twofold higher mortality.
Keywords: hemodialysis; geriatric nephrology; risk factors, Incidence; Proportional Hazards Models; kidney transplantation; Executive Function; renal dialysis; Alzheimer’s Disease; Medicare; Institutionalization; Registries
Introduction
Dementia, a state of persistent and progressive cognitive impairment (1), is the leading cause of disability and dependence worldwide (2–4). In the general population, Alzheimer’s disease is the most common dementia subtype, accounting for approximately one half of patients with dementia (1), and it is the sixth leading cause of death in the United States (5). As life expectancy increases, there is an exponential increase in both the prevalence and costs of dementia (4,6,7). Dementia and Alzheimer’s disease are associated with higher mortality (8–10), and it is projected that, by 2050, 1.6 million or 43% of older adult deaths will be due to Alzheimer’s disease. In public perception, dementia is the most feared age-related disorder resulting from the loss of independence and declining quality of life associated with this disorder (11). Furthermore, dementia complicates clinical shared decision-making processes, especially for life-sustaining treatments, like hemodialysis (12).
There has been a continuous increase in the number of older adults (age ≥65 years old) initiating hemodialysis in the United States, and in 2014, 49.6% of patients initiating hemodialysis were older adults (13). Additionally, patients undergoing hemodialysis are living longer; the number of patients on hemodialysis ages 85 years old and older has increased from 5073 in 1996 to 24,231 in 2014 (13).
These older patients on hemodialysis are likely at high risk of developing and being diagnosed with dementia and Alzheimer’s disease given that they have worse cognitive function than the general population (14,15), have a greater burden of white matter disease and cerebral atrophy (16), experience significant decline in cognitive function while undergoing hemodialysis (17), and have a high burden of cognitive impairment even at younger ages (17–19). Executive function is the domain of cognition most affected by hemodialysis within the first 2 years after initiation (18), and impairments in executive function are early signs of dementia and Alzheimer’s disease (20,21).
Among older patients with ESKD initiating hemodialysis, we sought to (1) estimate the risk of incident diagnosis of dementia and the Alzheimer’s disease subtype of dementia, (2) identify prehemodialysis predictors of incident diagnosis of all-cause dementia and the Alzheimer’s disease subtype of dementia, and (3) compare the risk of subsequent mortality between older patients on hemodialysis who were diagnosed with any dementia or the Alzheimer’s disease subtype of dementia and those who were not.
Materials and Methods
Study Population
We identified 608,988 older (age ≥66 years old) patients with ESKD in the US Renal Data System (USRDS), a national registry of all patients with ESKD in the United States, who initiated hemodialysis between January 1, 2001 and December 31, 2013. The USRDS included the Centers for Medicare and Medicaid Services (CMS) 2728 Medical Evidence Report Form and was linked to Medicare claims data. This study was limited to participants with Medicare as the primary payer as identified through the Medicare Enrollment Database. We excluded those without Medicare Part A and B (n=215,446) after hemodialysis initiation, those with prevalent diagnosis with dementia at the time of hemodialysis initiation on the basis of a 1-year look back (n=18,331), those with incomplete data or missing a Medical Evidence Report (n=16,928), and those without follow-up (n=1615). We ascertained patient characteristics, dialysis factors, and comorbid conditions from the Medical Evidence Report and USRDS data as well as a 1-year look back in Medicare claims data for 356,668 older patients with ESKD initiating hemodialysis (Supplemental Appendix 1). As is standard with USRDS data, mortality was augmented through linkage with the Social Security Death Master File and CMS data. This study was reviewed by the Johns Hopkins School of Medicine Institutional Review Board, and it was determined to qualify for an exemption under the Code of Federal Regulations, Protection of Human Subjects (45 CFR 46.10[b]), because the study participants cannot be identified directly or through linked identifiers.
International Classification of Disease–Reported Diagnosed Dementia and Alzheimer’s Disease
Similar to other studies (22–27), we identified incident diagnosis with dementia (331.0, 331.1, 331.2, 331.7, 290.0, 290.1, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 294.0, 294.1, 294.8 [294.20 after 2012], 797) and diagnosis with Alzheimer’s disease (331.0) using a validated International Classification of Disease 9 (ICD-9) code algorithm. A previous publication reported the sensitivity and specificity of these ICD-9 claims in Medicare claims data for older adults primarily without ESKD as 0.85 and 0.89, respectively, for dementia and 0.64 and 0.95, respectively, for Alzheimer’s disease (22). However, these claims have not been validated among patients with ESKD. Incident diagnosis with dementia after hemodialysis initiation was ascertained through a 1-year look back; patients with a claim for dementia in the year before hemodialysis initiation were excluded. Therefore, all results should be interpreted as documentation of a diagnosis with dementia rather than development of dementia.
Predictors of Diagnosis with Dementia and Alzheimer’s Disease
Potential predictors of dementia and Alzheimer’s disease diagnosis included patient characteristics (age, sex, race, ethnicity, body mass index, and employment status), dialysis factors (cause of ESKD, year of hemodialysis initiation, and eGFR≥15 ml/min per 1.73 m2), comorbidities (cancer, hypertension, diabetes, peripheral vascular disease, stroke/transient ischemic attack, atherosclerotic heart disease, alcohol dependence, and tobacco use), and measures of functional status (inability to ambulate, institutionalized, and needing activities of daily living assistance) from the 2728 form as well as a 1-year look back in Medicare claims data.
Statistical Analyses
Risk of Diagnosis with Dementia and Alzheimer’s Disease.
We used the Kaplan–Meier method to estimate the incidence rates of diagnoses with dementia and Alzheimer’s disease and Cox proportional hazard regression models to identify risk factors for incident diagnosis with dementia, censoring for end of follow-up (December 31, 2013), end of Medicare coverage, change in dialysis modality, kidney transplantation, or mortality. The final multivariable model was selected on the basis of optimal parsimony by minimizing the Akaike Information Criterion.
Competing Risk Model.
Estimating the cumulative incidence of diagnosis with dementia for older patients with ESKD initiating hemodialysis is complicated by the competing risks of death, change in the type of kidney replacement therapy, and kidney transplantation. Therefore, we estimated the cumulative incidence of diagnosis with dementia and associations between the predictors and diagnosis with dementia using a competing risks survival regression on the basis of Fine and Gray proportional subhazards model (28). Using this method, we estimated and plotted the cumulative incidence function (i.e., the risk of being diagnosed with dementia by a given time as we have previously published) (27). We then calculated the lifetime risk of diagnosis with dementia by sex using methods that were developed to estimate the lifetime risk of diagnosis with dementia in prospective cohort studies (29) (i.e., the risk of diagnosis with dementia after a patient initiates hemodialysis during his or her lifetime) accounting for mortality, change in dialysis modality, and kidney transplant. By using a competing risks approach, this method for estimating the standardized lifetime risk of diagnosis with dementia adjusts the cumulative incidence for the mortality, change in dialysis modality, and kidney transplant experience, and it is interpreted as the remaining lifetime risk of being diagnosed with dementia conditional on survival to dialysis initiation free of dementia diagnosis. This method accounts for both the higher incidence of diagnosis with dementia with age and the decrease in life expectancy seen with age, resulting in a relatively invariant estimate of the lifetime risk in older patients on hemodialysis (29). A total of 356,668 older patients with ESKD initiating hemodialysis free of dementia diagnosis were followed for up to 13 years to estimate the lifetime risk of diagnosis with dementia and Alzheimer’s disease. This estimate is interpreted as remaining lifetime risk of being diagnosed with dementia over 13 years of follow-up given dementia diagnosis-free survival to dialysis initiation adjusting for the competing risk of mortality, change in dialysis modality, and kidney transplantation. This measure provides a useful public health statistic for resource planning and patient education on the true risk of the disease (29). We used a similar approach to study diagnosis with Alzheimer’s disease.
Mortality after Diagnosis with Dementia and Alzheimer’s Disease.
We then estimated the hazard ratio (HR) of mortality associated with diagnosis with dementia using a Cox proportional hazard regression model. We considered diagnosis with dementia as time-varying exposure: older patients on hemodialysis contributed person time to the nondementia group until the diagnosis of dementia, and then, they contributed person time to the dementia group until mortality, change in kidney replacement therapy, kidney transplantation, or administrative censoring (September 16, 2015). We also tested whether the risk of mortality differed by age, sex, race, and diabetes; we pulled the stratified associations from the Cox proportional hazards models and estimated them using the lincom command in Stata. We used a similar approach to quantify the association between diagnosis with Alzheimer’s disease and mortality.
General Statistical Analyses.
Analyses were performed using Stata 14.0; remaining lifetime risk from the Practical Incidence Estimator macro (SAS 9.4) that was developed for the estimation of lifetime diagnosis with dementia risk using a competing risks framework in the Framingham cohort was performed as previously described (29). A significance level of 0.05 was used, and all hypotheses tested were two sided.
Results
Study Population
Among 356,668 older patients with ESKD initiating hemodialysis, mean age was 76 (SD=7) years old, 47% were women, and 20% were black. The median follow-up time was 1.3 years (interquartile range, 0.5–2.8 years; maximum =13 years); 63,303 (18%) were diagnosed with dementia during study follow-up, and of those with a diagnosis of dementia, 13,897 (22%) were diagnosed with Alzheimer’s disease subtype of dementia (Table 1). Characteristics stratified by age are presented in Supplemental Appendix 2. The incidence rate was 90.8 (95% confidence interval [95% CI], 90.1 to 91.5) per 1000 person-years for diagnosis with dementia and 18.2 (95% CI, 17.9 to 18.5) per 1000 person-years for diagnosis with Alzheimer’s disease. During follow-up, 239,203 deaths occurred (67%), and 6405 (2%) received kidney transplantation.
Table 1.
Characteristics of older (age ≥66 years old) patients with ESKD initiating hemodialysis by incident diagnosis with dementia and Alzheimer’s disease
| Characteristic, % | Total Population | No Dementia | Dementia | Alzheimer’s disease | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total, n=293,365 | Men, n=159,804 | Women, n=133,561 | Total, n=63,303 | Men, n=30,744 | Women, n=32,559 | Total, n=13,897 | Men, n=6607 | Women, n=7290 | ||
| Age, yr | ||||||||||
| 66–70 | 25 | 26 | 26 | 27 | 18 | 18 | 19 | 17 | 16 | 17 |
| 71–75 | 25 | 26 | 26 | 26 | 23 | 23 | 24 | 24 | 22 | 25 |
| 76–80 | 24 | 23 | 24 | 23 | 26 | 26 | 25 | 28 | 29 | 26 |
| 81–85 | 17 | 16 | 17 | 16 | 21 | 22 | 21 | 21 | 21 | 21 |
| >85 | 9 | 8 | 8 | 8 | 12 | 12 | 12 | 11 | 11 | 11 |
| Age, yr, mean (SD) | 76 (7) | 76 (7) | 76 (7) | 76 (7) | 77 (7) | 77 (7) | 77 (7) | 77 (6) | 78 (6) | 77 (6) |
| Women | 47 | 46 | — | — | 51 | — | — | 53 | — | — |
| Race | ||||||||||
| White | 75 | 77 | 81 | 72 | 66 | 71 | 60 | 64 | 71 | 58 |
| Black | 20 | 18 | 15 | 22 | 30 | 24 | 35 | 31 | 25 | 37 |
| Other/unknown | 5 | 5 | 4 | 6 | 5 | 5 | 5 | 5 | 4 | 5 |
| Hispanic ethnicity | 10 | 9 | 9 | 10 | 11 | 11 | 11 | 14 | 13 | 15 |
| Employment status | ||||||||||
| Retired | 75 | 75 | 80 | 69 | 74 | 80 | 68 | 74 | 81 | 67 |
| Employed | 2 | 2 | 3 | 1 | 0.9 | 1 | 0.4 | 0.8 | 1 | 0.4 |
| Unemployed | 8 | 8 | 6 | 12 | 9 | 7 | 11 | 9 | 7 | 11 |
| Other | 16 | 15 | 11 | 20 | 16 | 11 | 20 | 17 | 11 | 22 |
| Cause of ESKD | ||||||||||
| Glomerular diseases | 6 | 6 | 6 | 5 | 5 | 5 | 4 | 5 | 5 | 4 |
| Diabetes | 43 | 43 | 40 | 46 | 44 | 41 | 47 | 44 | 41 | 47 |
| Hypertension | 36 | 35 | 36 | 34 | 39 | 40 | 38 | 40 | 41 | 39 |
| Other causes | 16 | 16 | 18 | 14 | 13 | 14 | 11 | 12 | 14 | 10 |
| Body mass index, kg/m2 | ||||||||||
| <18.5 | 4 | 4 | 3 | 5 | 5 | 4 | 6 | 5 | 3 | 6 |
| 18.5–25.0 | 37 | 36 | 39 | 33 | 41 | 44 | 38 | 42 | 45 | 40 |
| 25.0–30.0 | 30 | 31 | 33 | 27 | 30 | 33 | 28 | 31 | 34 | 28 |
| ≥30.0 | 28 | 29 | 25 | 35 | 24 | 19 | 28 | 22 | 18 | 26 |
| Body mass index, kg/m2, mean (SD) | 27.3 (6.4) | 27.5 (6.5) | 26.9 (5.7) | 28.3 (7.2) | 26.6 (6.0) | 26.0 (5.3) | 27.1 (6.6) | 26.3 (5.9) | 25.9 (5.2) | 26.7 (6.4) |
| eGFR≥15, ml/min per 1.73 m2 | 11 | 12 | 14 | 9 | 9 | 11 | 7 | 8 | 11 | 6 |
| eGFR, mean (SD) | 9.6 (4.5) | 9.7 (4.6) | 10.2 (4.8) | 9.0 (4.1) | 9.2 (4.2) | 9.9 (4.5) | 8.6 (3.9) | 9.0 (4.1) | 9.7 (4.3) | 8.4 (3.8) |
| Cancer | 15 | 15 | 18 | 12 | 12 | 16 | 9 | 12 | 16 | 9 |
| Hypertension | 90 | 90 | 89 | 92 | 91 | 90 | 93 | 92 | 91 | 93 |
| Diabetes | 60 | 60 | 57 | 63 | 60 | 56 | 64 | 60 | 56 | 64 |
| Peripheral vascular disease | 19 | 19 | 21 | 17 | 17 | 20 | 15 | 16 | 18 | 14 |
| Stroke/transient ischemic attack | 14 | 13 | 13 | 14 | 19 | 19 | 19 | 18 | 18 | 18 |
| Atherosclerotic heart disease | 46 | 47 | 50 | 43 | 44 | 47 | 41 | 41 | 45 | 38 |
| Congestive heart failure | 41 | 42 | 41 | 42 | 39 | 38 | 40 | 37 | 36 | 38 |
| Chronic obstructive pulmonary disease | 13 | 13 | 14 | 12 | 10 | 12 | 9 | 9 | 11 | 8 |
| Drug dependence | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 |
| Alcohol dependence | 1 | 1 | 2 | 0.5 | 1 | 2 | 0.5 | 0.7 | 1 | 0.3 |
| Tobacco use | 7 | 7 | 8 | 6 | 6 | 8 | 5 | 6 | 7 | 5 |
| Inability to ambulate | 10 | 9 | 8 | 10 | 12 | 11 | 14 | 11 | 9 | 12 |
| Institutionalized | 15 | 14 | 12 | 17 | 20 | 18 | 22 | 17 | 15 | 19 |
| Activities of daily living assistance | 29 | 28 | 24 | 32 | 33 | 29 | 37 | 32 | 28 | 36 |
| Year of hemodialysis initiation | ||||||||||
| 2001–2004 | 32 | 32 | 31 | 34 | 34 | 33 | 36 | 32 | 36 | 38 |
| 2005–2007 | 24 | 24 | 24 | 24 | 26 | 26 | 26 | 27 | 29 | 27 |
| 2008–2010 | 23 | 22 | 23 | 21 | 25 | 25 | 24 | 23 | 24 | 23 |
| 2011–2013 | 21 | 22 | 23 | 22 | 16 | 16 | 15 | 13 | 12 | 12 |
Alzheimer’s disease is a subtype of dementia; therefore, the patients with Alzheimer’s disease are a subset of the patients with dementia. Dementia and Alzheimer’s disease are International Classification of Disease–reported diagnoses of dementia and Alzheimer’s disease, respectively. —, not applicable.
Risk of Diagnoses with Dementia and Alzheimer’s Disease after Hemodialysis Initiation
Within the first year after initiating hemodialysis, 4.6% of older women and 3.7% of older men were diagnosed with dementia; the corresponding risks of diagnosis with Alzheimer’s disease were 0.6% and 0.4%, respectively. The remaining lifetime risks of diagnosis with dementia after accounting for the competing risks of death, change in dialysis modality and transplantation for older patients initiating hemodialysis were 25% for women and 21% for men (Table 2). The corresponding lifetime risks of diagnosis with Alzheimer’s disease for older patients initiating hemodialysis were 5.4% for women and 4.2% for men (Table 2).
Table 2.
The risk of diagnosis with dementia and Alzheimer’s disease after hemodialysis initiation by sex
| Time Frame | Dementia | Alzheimer’s Disease | ||
|---|---|---|---|---|
| Women, % (95% CI) | Men, % (95% CI) | Women, % (95% CI) | Men, % (95% CI) | |
| 1 yr | 4.6 (4.2 to 5.1) | 3.7 (3.4 to 4.1) | 0.6 (0.4 to 0.8) | 0.4 (0.3 to 0.5) |
| 5 yr | 16 (15 to 16) | 13 (13 to 14) | 2.6 (2.3 to 2.9) | 2.0 (1.8 to 2.2) |
| 10 yr | 22 (22 to 23) | 19 (18 to 19) | 4.3 (3.9 to 4.6) | 3.4 (3.0 to 3.6) |
| Lifetime | 25 (24 to 26) | 21 (20 to 22) | 5.4 (4.0 to 5.6) | 4.2 (3.4 to 4.4) |
The lifetime risks were estimated for 356,668 older patients with ESKD using a competing risks survival analysis. The lifetime risk is interpreted as remaining lifetime risk of being diagnosed with dementia (or Alzheimer’s disease) over 13 years of follow-up given dementia diagnosis-free survival to dialysis initiation and accounting for the competing risks of death, change in dialysis modality, and kidney transplantation. 95% CI, 95% confidence interval.
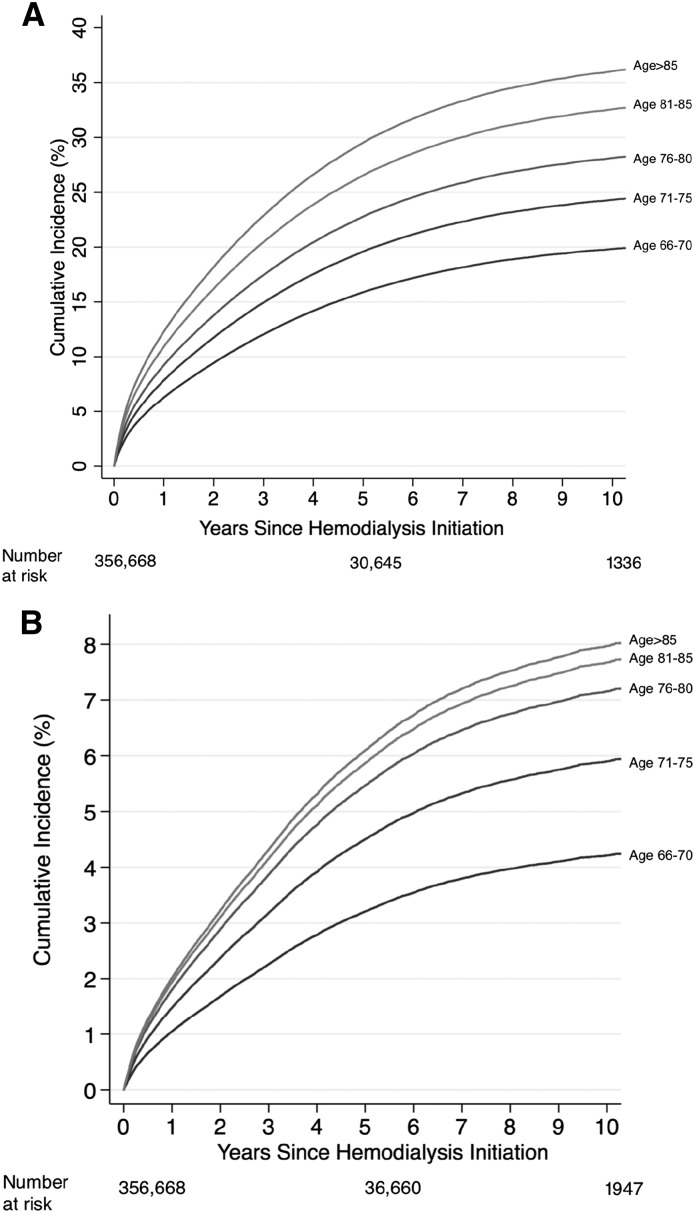
The 10-year risks of posthemodialysis dementia diagnosis were 20% for patients on hemodialysis ages 66–70 years old, 24% for those ages 71–75 years old, 28% for those ages 76–80 years old, 34% for those ages 81–85 years old, and 36% for those older than 85 years old (Figure 1A). Similarly, the risk of Alzheimer’s disease diagnosis was higher with older age at hemodialysis initiation (Figure 1B). The 10-year risks of diagnosis with Alzheimer’s disease were 4.3% for patients on hemodialysis ages 66–70 years old, 5.9% for those ages 71–75 years old, 7.1% for those ages 76–80 years old, 7.6% for those ages 81–85 years old, and 8.0% for those older than 85 years old.
Figure 1.
The risk of diagnosis with dementia and Alzheimer's disease increased with age at hemodialysis initiation. The risk (cumulative incidence) was estimated for 356,668 older patients with ESKD using a competing risks survival analysis. Dementia represents the International Classification of Disease (ICD)–reported diagnoses of dementia. (A) Alzheimer’s disease is a subtype of dementia; therefore, the patients with Alzheimer’s disease are a subset of the patients with dementia. (B) Alzheimer’s disease represents the ICD-reported diagnoses of Alzheimer’s disease. Cumulative incidence of diagnosis with dementia. Cumulative incidence of diagnosis with Alzheimer’s disease.
Risk Factors for Dementia and Alzheimer’s Disease Diagnoses
Patients on hemodialysis who were diagnosed with dementia were older at the time of hemodialysis initiation (81–85 years old: 21% versus 16%; >85 years old: 12% versus 8%; P<0.001), more likely to be women (51% versus 46%; P<0.001), more likely to have had stroke/transient ischemic attack (19% versus 13%; P<0.001), more likely to have an inability to ambulate (12% versus 9%; P<0.001), more likely to be institutionalized (20% versus 14%; P<0.001), and more likely to need assistance with activities of daily living (33% versus 28%; P<0.001) (Table 1). The risk of dementia diagnosis after hemodialysis initiation was higher with older age at initiation (Table 3). Additionally, black patients on hemodialysis (HR, 1.70; 95% CI, 1.67 to 1.73), underweight patients (body mass index <18.5 kg/m2; HR, 1.29; 95% CI, 1.24 to 1.35), patients with a history of stroke/transient ischemic attack (HR, 1.43; 95% CI, 1.40 to 1.46), patients with activities of daily living dependence (HR, 1.09; 95% CI, 1.07 to 1.11), and institutionalized patients (HR, 1.36; 95% CI, 1.33 to 1.39) were at higher risk of dementia diagnosis after initiation. Additionally, compared with those who initiated hemodialysis between 2001 and 2005, those who initiated hemodialysis between 2008 and 2010 had a 1.17-fold higher risk of dementia diagnosis (95% CI, 1.14 to 1.19), and those who initiated hemodialysis between 2011 and 2013 had a 1.31-fold (95% CI, 1.27 to 1.34) higher risk of dementia diagnosis.
Table 3.
Risk factors for incident diagnosis with dementia and Alzheimer’s disease in older (age ≥66 years old) patients with ESKD initiating hemodialysis
| Risk Factor | Dementia Hazard Ratios (95% CI) | Alzheimer’s Disease Hazard Ratios (95% CI) |
|---|---|---|
| Age, yr | ||
| 66–70 | Reference | Reference |
| 71–75 | 1.31 (1.28 to 1.34) | 1.47 (1.40 to 1.56) |
| 76–80 | 1.59 (1.55 to 1.63) | 1.86 (1.76 to 1.96) |
| 81–85 | 1.91 (1.86 to 1.96) | 2.04 (1.93 to 2.16) |
| ≥86 | 2.11 (2.04 to 2.18) | 2.11 (1.97 to 2.25) |
| Women | 1.10 (1.08 to 1.12) | 1.12 (1.08 to 1.16) |
| Race | ||
| White | Reference | Reference |
| Black | 1.70 (1.67 to 1.73) | 1.78 (1.71 to 1.85) |
| Other/unknown | 1.01 (0.98 to 1.05) | 0.94 (0.87 to 1.02) |
| Hispanic ethnicity | 1.30 (1.26 to 1.33) | 1.75 (1.66 to 1.84) |
| Employment status | ||
| Retired | Reference | Reference |
| Employed | 0.68 (0.63 to 0.74) | 0.68 (0.56 to 0.81) |
| Unemployed | 1.08 (1.05 to 1.11) | 0.99 (0.94 to 1.06) |
| Other | 1.05 (1.02 to 1.07) | 1.08 (1.03 to 1.13) |
| Body mass index, kg/m2 | ||
| <18.5 | 1.29 (1.24 to 1.35) | 1.31 (1.20 to 1.43) |
| 18.5–25.0 | 1.27 (1.24 to 1.29) | 1.35 (1.29 to 1.42) |
| 25.0–30.0 | 1.17 (1.15 to 1.20) | 1.24 (1.19 to 1.30) |
| ≥30.0 | Reference | Reference |
| eGFR<15, ml/min per 1.73 m2 | 1.17 (1.14 to 1.21) | 1.18 (1.11 to 1.26) |
| Cancer | 0.80 (0.78 to 0.82) | 0.81 (0.77 to 0.85) |
| Hypertension | 1.05 (1.02 to 1.08) | 1.13 (1.06 to 1.20) |
| Diabetes | 1.07 (1.05 to 1.08) | 1.05 (1.02 to 1.09) |
| Peripheral vascular disease | 0.88 (0.86 to 0.90) | 0.84 (0.80 to 0.88) |
| Stroke/transient ischemic attack | 1.43 (1.40 to 1.46) | 1.28 (1.22 to 1.34) |
| Atherosclerotic heart disease | 0.90 (0.88 to 0.91) | 0.85 (0.82 to 0.89) |
| Congestive heart failure | 0.86 (0.84 to 0.87) | 0.84 (0.81 to 0.87) |
| Alcohol dependence | 1.06 (0.98 to 1.15) | 0.80 (0.65 to 0.97) |
| Tobacco use | 1.01 (0.98 to 1.04) | 0.96 (0.90 to 1.04) |
| Inability to ambulate | 1.19 (1.15 to 1.22) | 1.08 (1.01 to 1.14) |
| Institutionalized | 1.36 (1.33 to 1.39) | 1.10 (1.05 to 1.15) |
| Activities of daily living assistance | 1.09 (1.07 to 1.11) | 1.12 (1.07 to 1.16) |
| Year of hemodialysis initiation | ||
| 2001–2005 | Reference | Reference |
| 2005–2007 | 1.04 (1.02 to 1.06) | 1.08 (1.04 to 1.13) |
| 2008–2010 | 1.17 (1.14 to 1.19) | 1.05 (1.00 to 1.10) |
| 2011–2013 | 1.31 (1.27 to 1.34) | 0.93 (0.88 to 0.98) |
All hazard ratios are from a single adjusted competing risks survival model for the diagnoses with dementia and Alzheimer’s disease outcomes separately. Alzheimer’s disease is a subtype of dementia; therefore, the patients with Alzheimer’s disease are a subset of the patients with dementia. Dementia and Alzheimer’s disease are International Classification of Disease–reported diagnoses of dementia or Alzheimer’s disease. 95% CI, 95% confidence interval.
Although most predictors of diagnosis with Alzheimer’s disease were similar to those identified for diagnosis with dementia, there were some notable differences in the strength of the associations (Table 3). Hispanic patients on hemodialysis had a 1.75-fold (95% CI, 1.66 to 1.84) higher risk of diagnosis with Alzheimer’s disease. In addition, compared with those who initiated hemodialysis between 2001 and 2005, those who initiated hemodialysis between 2005 and 2007 had a 1.08-fold (95% CI, 1.04 to 1.13) higher risk of diagnosis with Alzheimer’s disease, and those who initiated hemodialysis between 2008–2010 had a 1.05-fold (95% CI, 1.00 to 1.10) higher risk of diagnosis with Alzheimer’s disease; however, those who initiated hemodialysis between 2011 and 2013 had a decreased risk (HR, 0.93; 95% CI, 0.88 to 0.98).
As a sensitivity analysis, we estimated these associations using a Cox proportional hazards model; as expected, treating the deaths, change in dialysis modality, and transplantation as censored observations in a traditional Cox model inflates the estimate of the cumulative incidence.
Mortality after Posthemodialysis Dementia Diagnosis
The risk of mortality was higher among older patients on hemodialysis who were diagnosed with dementia after initiation (Table 4). Older patients on hemodialysis with a dementia diagnosis were at 2.14-fold (95% CI, 2.07 to 2.22) higher risk of mortality compared with older patients on hemodialysis without a dementia diagnosis, independent of patient, dialysis, and comorbidity factors as well as measures of functional status. Additionally, the association between diagnosis with dementia and mortality differed by race (P value for interaction =0.04) and diabetes status (P value for interaction =0.001) (Table 4), such that the risk of mortality associated with dementia diagnosis was greater for black patients and patients without diabetes.
Table 4.
The association of posthemodialysis diagnosis with dementia and Alzheimer’s disease on mortality stratified by age, race, sex, and diabetes status
| Stratification Factor | Dementia Hazard Ratios (95% CI) | Alzheimer’s Disease Hazard Ratios (95% CI) |
|---|---|---|
| Overall risk of mortality | 2.14 (2.07 to 2.22) | 2.01 (1.89 to 2.15) |
| Age, yr | ||
| 66–74 | 2.17 (2.05 to 2.29) | 2.00 (1.79 to 2.22) |
| ≥75 | 2.17 (2.08 to 2.26) | 2.06 (1.85 to 1.94) |
| P value for interaction | 0.99 | 0.63 |
| Race | ||
| Nonblack | 2.10 (2.02 to 2.18) | 1.97 (1.82 to 2.13) |
| Black | 2.26 (2.12 to 2.41) | 2.09 (1.87 to 2.34) |
| P value for interaction | 0.04 | 0.40 |
| Sex | ||
| Men | 2.15 (2.05 to 2.26) | 2.10 (1.91 to 2.30) |
| Women | 2.13 (2.03 to 2.23) | 1.85 (1.78 to 2.13) |
| P value for interaction | 0.76 | 0.25 |
| Diabetes | ||
| No | 2.29 (2.18 to 2.41) | 2.31 (1.09 to 2.55) |
| Yes | 2.05 (1.96 to 2.14) | 1.85 (1.70 to 2.01) |
| P value for interaction | 0.001 | 0.001 |
All models are adjusted for the patient, dialysis, and comorbidity factors as well as measures of functional status listed in Table 3. The hazard ratios are estimated using a Cox proportional hazards model with time-varying diagnoses with dementia and Alzheimer’s disease. Alzheimer’s disease is a subtype of dementia; therefore, the patients with Alzheimer’s disease are a subset of the patients with dementia. Dementia and Alzheimer’s disease are International Classification of Disease–reported diagnoses of dementia or Alzheimer’s disease. 95% CI, 95% confidence interval.
Mortality after Posthemodialysis Alzheimer’s Disease Diagnosis
The risk of mortality was higher among older patients on hemodialysis who were diagnosed with Alzheimer’s disease after initiation (Table 4). Older patients on hemodialysis with a diagnosis of Alzheimer’s disease were at 2.01-fold (95% CI, 1.89 to 2.15) higher risk of mortality compared with older patients on hemodialysis without a diagnosis of Alzheimer’s disease, independent of patient, dialysis, and comorbidity factors as well as measures of functional status. The association between Alzheimer’s disease diagnosis and mortality was greater for patients without diabetes (P value for interaction =0.001) (Table 4).
Discussion
In this national study of 356,668 older patients with ESKD initiating hemodialysis, within the first year of hemodialysis initiation, 4.6% of older women and 3.7% of older men were diagnosed with dementia. The remaining lifetime risks of diagnosis with dementia accounting for the competing risks of death, change in dialysis modality, and kidney transplantation for older patients initiating hemodialysis were 25% for women and 21% for men; the corresponding remaining lifetime risks of diagnosis with Alzheimer’s disease were 5.4% and 4.2%, respectively. Patient characteristics and measures of functional status were independently associated with a greater risk of dementia and Alzheimer’s disease diagnoses. Older patients on hemodialysis who were diagnosed with dementia were subsequently at a twofold higher risk of death; a similar mortality risk was observed for older patients on hemodialysis who were diagnosed with Alzheimer’s disease.
Previous findings from the Framingham study suggest that the 10-year incidence of dementia is 1%–1.5% in adults age 65 years old and 7.4%–7.6% in adults age 75 years old (30). Using a similar analytic approach to account for the competing risk of mortality and kidney transplant, we found that the 10-year risk of diagnosed posthemodialysis dementia was 19% for patients with ESKD ages 66–70 years old and that it rose to 28% for those 76–80 years of age. Similarly, our observed 10-year incidence of diagnosed Alzheimer’s disease after hemodialysis initiation of 4.3% in those ages 66–70 years old, which rose to 7.1% for those 76–81 years old, is greater than the findings from the Framingham study (0.6%–0.9% incidence in adults age 65 years old and 4.4%–5.4% incidence in adults age 75 years old). Additionally, we found that there was a higher risk of diagnosis of dementia over time. These findings could represent a true increase in patients with new cases of dementia and Alzheimer’s disease (until 2011) or could be due to changes in billing code practices over time. Additionally, age is the strongest risk factor for dementia diagnosis. The higher risk of diagnosis of dementia may be due to the increase in the number of older (ages 75 years old and older) patients initiating hemodialysis since 2001; however, our results were adjusted for age.
The estimates from the Framingham study included patients with both diagnosed and undiagnosed cases. These differences in defining patients with dementia and patients with Alzheimer’s disease are not trivial given that a previous validation study suggested that only one half of those who would meet diagnostic criteria for dementia receive a physician diagnosis of dementia (31). Additionally, with this estimated rate of underdiagnosis in mind, the risk of diagnoses with dementia and Alzheimer’s disease after hemodialysis initiation compared with in the general older adult population is potentially even more substantial than what comparing incidence rates would suggest. Additionally, we identified black and Hispanic patients on hemodialysis as being at an elevated risk of being diagnosed with dementia, which is consistent with findings from the geriatric literature (32); these racial and ethnic differences have been attributed to the greater burden of chronic comorbidity in older adults (33,34) as well as older adults with ESKD (35). It is also possible that these black and Hispanic patients on hemodialysis are now receiving routine medical care, which allows for the diagnosis with dementia after hemodialysis initiation, because dementia is often undiagnosed in this population. This work builds on the previous findings about prevalent dementia among patients on hemodialysis (14,15,36,37) and extends these findings to incident dementia among older patients with ESKD initiating hemodialysis.
The potential mechanisms of dementia diagnosis after hemodialysis initiation are not clear, although the prevailing hypothesis is that cognitive impairment manifests in patients undergoing hemodialysis as part of a microvascular process. Among older patients on hemodialysis, dementia likely exists on a spectrum, influenced by both vascular processes as well as the Alzheimer’s disease pathology, although the former process is likely more common (38–41).
Similar to findings from studies of older adults (8–10) and patients undergoing dialysis (36,37,42), we found that there is a great burden of mortality among those diagnosed with dementia or Alzheimer’s disease. Among patients with ESKD of all ages who had a diagnosis of dementia before hemodialysis initiation, there was a 1.87-fold higher risk of postdialysis mortality (42), and a study of patients on hemodialysis of all ages found that prevalent dementia is associated with a 1.48-fold higher risk of mortality (36). Our findings suggest that the risk of dementia diagnosis is highest among those with activities of daily living disability, which may be one pathway linking dementia and mortality; furthermore, the diagnosis of Alzheimer’s disease requires the impairment in functioning. Mortality can result among patients on hemodialysis with a diagnosis of dementia as the result of an inability to perform self-care tasks, to consume adequate nutritional intake, to adhere to complex diet and medication regimens, to identify personal safety issues, or to communicate new symptoms or infections, particularly pneumonia (43,44). Additionally, the relationship between poor executive function and mortality among patients on hemodialysis is partially explained by cardiovascular disease burden (38).
This study has a few notable limitations. To identify patients with incident cases of dementia after hemodialysis initiation, we had to limit our population to Medicare-primary patients on hemodialysis with 1 year of claims before hemodialysis initiation, a criterion that could differentially affect those under 66 years old and thus, the generalizability to younger cohorts. However, given that all patients with ESKD requiring dialysis therapy are eligible for Medicare, this is a common inclusion criterion in studies of patients with ESKD (45–47). Additionally, this national registry does not capture important metrics of aging, like frailty and measured cognitive function (15,48,49), which may be important predictors of incident diagnosis with dementia in patients on hemodialysis; however, we were able to study functional dependence as measured by activities of daily living, which is an important metric of aging in patients on hemodialysis (50). Also, this national registry does not include patients with CKD; therefore, we are unable to estimate the effect of initiating dialysis on the risk of diagnosis with dementia. Also, the distinction between the general category of diagnosis with “dementia” and the specific diagnosis of “Alzheimer’s disease” is challenging in real world clinical settings reflected in Medicare claims data, and thus, it is likely that the diagnosis of activities of daily living is an underestimate and reflects coding habits. Alzheimer’s disease cannot be truly diagnosed without a postmortem pathologic confirmation, and it likely that the claims-based diagnosis of Alzheimer’s disease more likely reflects a physician’s suspected diagnosis, which may be reflected in the percentage of all patients with dementia diagnosed with Alzheimer’s disease. In fact, dementia diagnoses are likely an underestimate of the true burden of dementia, because we relied on diagnoses code for dementia rather than a neurocognitive battery (31). Furthermore, the more clinical encounters a patient has with the health care system, particularly encounters beyond the dialysis unit, the more likely they are to be given a diagnosis of dementia and the more likely they are to die. Furthermore, it is challenging to distinguish patients with incident cases of dementia from those with prevalent or preexisting dementia in administrative diagnosis claims. For this reason, we have focused on incident diagnoses of dementia and recognize that dementia onset likely occurred before diagnosis. Finally, our study was on the basis of observational data, and thus, it remains unclear whether preventing dementia or Alzheimer’s disease would improve the high rates of mortality among older patients with ESKD initiating hemodialysis.
In conclusion, the risk of diagnosed dementia and Alzheimer’s disease among older patients with ESKD initiating hemodialysis is substantially greater than the previously published risks of dementia and Alzheimer’s disease among community-dwelling older adults . A number of patient, dialysis, and comorbidity factors as well as measures of functional status were associated with dementia and Alzheimer’s disease diagnoses in this population. Older patients on hemodialysis who are diagnosed with dementia and Alzheimer’s disease are at twice the risk of subsequent mortality.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grants R01AG055781 (principal investigator [PI]: M.A.M.-D.), R01DK114074 (PI: M.A.M.-D.), K01AG043501 (PI: M.A.M.-D.), K01AG050699 (PI: A.L.G.), R01AG042504 (PI: D.L.S.), and K24DK101828 (PI: D.L.S.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice, “Dementia in Dialysis: An Eye on Best Practices,” on pages 1305–1306.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10150917/-/DCSupplemental.
References
- 1.Querfurth HW, LaFerla FM: Alzheimer’s’s disease. N Engl J Med 362: 329–344, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Sousa RM, Ferri CP, Acosta D, Albanese E, Guerra M, Huang Y, Jacob KS, Jotheeswaran AT, Rodriguez JJ, Pichardo GR, Rodriguez MC, Salas A, Sosa AL, Williams J, Zuniga T, Prince M: Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: A 10/66 Dementia Research Group population-based survey. Lancet 374: 1821–1830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sousa RM, Ferri CP, Acosta D, Guerra M, Huang Y, Jacob K, Jotheeswaran A, Hernandez MA, Liu Z, Pichardo GR, Rodriguez JJ, Salas A, Sosa AL, Williams J, Zuniga T, Prince M: The contribution of chronic diseases to the prevalence of dependence among older people in Latin America, China and India: A 10/66 Dementia Research Group population-based survey. BMC Geriatr 10: 53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harwood RH, Sayer AA, Hirschfeld M: Current and future worldwide prevalence of dependency, its relationship to total population, and dependency ratios. Bull World Health Organ 82: 251–258, 2004 [PMC free article] [PubMed] [Google Scholar]
- 5.US National Center for Health Statistics : Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities, Hyattsville, MD, US National Center for Health Statistics, 2016 [PubMed] [Google Scholar]
- 6.Hurd MD, Martorell P, Langa K: Future monetary costs of dementia in the United States under alternative dementia prevalence scenarios. J Popul Ageing 8: 101–112, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM: Monetary costs of dementia in the United States. N Engl J Med 368: 1326–1334, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrighi HM, Neumann PJ, Lieberburg IM, Townsend RJ: Lethality of Alzheimer’s disease and its impact on nursing home placement. Alzheimer’s Dis Assoc Disord 24: 90–95, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Weuve J, Hebert LE, Scherr PA, Evans DA: Deaths in the United States among persons with Alzheimer’s’s disease (2010-2050). Alzheimer’ss Dement 10: e40–e46, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA: Contribution of Alzheimer’s disease to mortality in the United States. Neurology 82: 1045–1050, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blazer DG, Yaffe K, Karlawish J: Cognitive aging: A report from the Institute of Medicine. JAMA 313: 2121–2122, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Miura Y, Asai A, Matsushima M, Nagata S, Onishi M, Shimbo T, Hosoya T, Fukuhara S: Families’ and physicians’ predictions of dialysis patients’ preferences regarding life-sustaining treatments in Japan. Am J Kidney Dis 47: 122–130, 2006 [DOI] [PubMed] [Google Scholar]
- 13.United States Renal Data System : 2016 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2016 [Google Scholar]
- 14.O’Lone E, Connors M, Masson P, Wu S, Kelly PJ, Gillespie D, Parker D, Whiteley W, Strippoli GF, Palmer SC, Craig JC, Webster AC: Cognition in people with end-stage kidney disease treated with hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 67: 925–935, 2016 [DOI] [PubMed] [Google Scholar]
- 15.McAdams-DeMarco MA, Tan J, Salter ML, Gross A, Meoni LA, Jaar BG, Kao WH, Parekh RS, Segev DL, Sozio SM: Frailty and cognitive function in incident hemodialysis patients. Clin J Am Soc Nephrol 10: 2181–2189, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drew DA, Bhadelia R, Tighiouart H, Novak V, Scott TM, Lou KV, Shaffi K, Weiner DE, Sarnak MJ: Anatomic brain disease in hemodialysis patients: A cross-sectional study. Am J Kidney Dis 61: 271–278, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drew DA, Weiner DE, Tighiouart H, Duncan S, Gupta A, Scott T, Sarnak MJ: Cognitive decline and its risk factors in prevalent hemodialysis patients. Am J Kidney Dis 69: 780–787, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurella Tamura M, Vittinghoff E, Hsu CY, Tam K, Seliger SL, Sozio S, Fischer M, Chen J, Lustigova E, Strauss L, Deo R, Go AS, Yaffe K; CRIC Study Investigators : Loss of executive function after dialysis initiation in adults with chronic kidney disease. Kidney Int 91: 948–953, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AJ, Kane RL: Cognitive impairment in hemodialysis patients is common. Neurology 67: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Albert MS, Moss MB, Tanzi R, Jones K: Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc 7: 631–639, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Kray J, Lindenberger U: Adult age differences in task switching. Psychol Aging 15: 126–147, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Taylor DH Jr., Østbye T, Langa KM, Weir D, Plassman BL: The accuracy of Medicare claims as an epidemiological tool: The case of dementia revisited. J Alzheimer’ss Dis 17: 807–815, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerra C, Linde-Zwirble WT, Wunsch H: Risk factors for dementia after critical illness in elderly Medicare beneficiaries. Crit Care 16: R233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Gao S, Hendrie HC, Kesterson J, Campbell NL, Shekhar A, Callahan CM: Antidepressant use in the elderly is associated with an increased risk of dementia. Alzheimer’s Dis Assoc Disord 30: 99–104, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfgram DF, Szabo A, Murray AM, Whittle J: Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int 35: 189–198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutcher SK, Rattinger GB, Langenberg P, Chhabra PT, Liu X, Rosenberg PB, Leoutsakos JM, Simoni-Wastila L, Walker LD, Franey CS, Zuckerman IH: Effect of medications on physical function and cognition in nursing home residents with dementia. J Am Geriatr Soc 62: 1046–1055, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAdams-DeMarco MA, Bae S, Chu N, Gross AL, Brown CH 4th, Oh E, Rosenberg P, Neufeld KJ, Varadhan R, Albert M, Walston J, Segev DL: Dementia and Alzheimer’s’s disease among older kidney transplant recipients. J Am Soc Nephrol 28: 1575–1583, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 29.Beiser A, D’Agostino RB Sr., Seshadri S, Sullivan LM, Wolf PA: Computing estimates of incidence, including lifetime risk: Alzheimer’s’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med 19: 1495–1522, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA: The lifetime risk of stroke: Estimates from the Framingham Study. Stroke 37: 345–350, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN; U.S. Preventive Services Task Force : Screening for dementia in primary care: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 138: 927–937, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Potter GG, Plassman BL, Burke JR, Kabeto MU, Langa KM, Llewellyn DJ, Rogers MA, Steffens DC: Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimer’ss Dement 5: 445–453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB: Dementia and Alzheimer’s disease incidence: A prospective cohort study. Arch Neurol 59: 1737–1746, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB: Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 29: 125–132, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers OB, Adams C, Rohrscheib MR, Servilla KS, Miskulin D, Bedrick EJ, Zager PG: Age, race, diabetes, blood pressure, and mortality among hemodialysis patients. J Am Soc Nephrol 21: 1970–1978, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurella M, Mapes DL, Port FK, Chertow GM: Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 21: 2543–2548, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Cohen LM, Ruthazer R, Moss AH, Germain MJ: Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol 5: 72–79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drew DA, Weiner DE, Tighiouart H, Scott T, Lou K, Kantor A, Fan L, Strom JA, Singh AK, Sarnak MJ: Cognitive function and all-cause mortality in maintenance hemodialysis patients. Am J Kidney Dis 65: 303–311, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarnak MJ, Tighiouart H, Scott TM, Lou KV, Sorensen EP, Giang LM, Drew DA, Shaffi K, Strom JA, Singh AK, Weiner DE: Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology 80: 471–480, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seidel UK, Gronewold J, Volsek M, Todica O, Kribben A, Bruck H, Hermann DM: The prevalence, severity, and association with HbA1c and fibrinogen of cognitive impairment in chronic kidney disease. Kidney Int 85: 693–702, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Drew DA, Weiner DE: Cognitive impairment in chronic kidney disease: Keep vascular disease in mind. Kidney Int 85: 505–507, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rakowski DA, Caillard S, Agodoa LY, Abbott KC: Dementia as a predictor of mortality in dialysis patients. Clin J Am Soc Nephrol 1: 1000–1005, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Burns A, Jacoby R, Luthert P, Levy R: Cause of death in Alzheimer’s’s disease. Age Ageing 19: 341–344, 1990 [DOI] [PubMed] [Google Scholar]
- 44.Brunnström HR, Englund EM: Cause of death in patients with dementia disorders. Eur J Neurol 16: 488–492, 2009 [DOI] [PubMed] [Google Scholar]
- 45.McAdams-Demarco MA, Grams ME, King E, Desai NM, Segev DL: Sequelae of early hospital readmission after kidney transplantation. Am J Transplant 14: 397–403, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobbels F, Skeans MA, Snyder JJ, Tuomari AV, Maclean JR, Kasiske BL: Depressive disorder in renal transplantation: An analysis of Medicare claims. Am J Kidney Dis 51: 819–828, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Grams ME, McAdams Demarco MA, Kucirka LM, Segev DL: Recipient age and time spent hospitalized in the year before and after kidney transplantation. Transplantation 94: 750–756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, Walston JD, Segev DL: Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 61: 896–901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McAdams-DeMarco MA, Suresh S, Law A, Salter ML, Gimenez LF, Jaar BG, Walston JD, Segev DL: Frailty and falls among adult patients undergoing chronic hemodialysis: A prospective cohort study. BMC Nephrol 14: 224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McAdams-Demarco MA, Law A, Garonzik-Wang JM, Gimenez L, Jaar BG, Walston JD, Segev DL: Activity of daily living disability and dialysis mortality: Better prediction using metrics of aging. J Am Geriatr Soc 60: 1981–1982, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.