Figure 2.
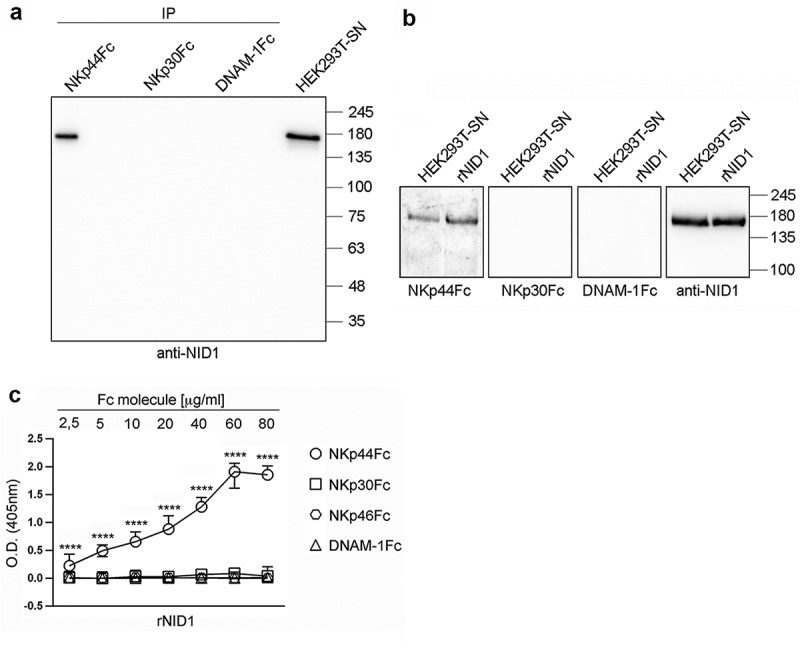
Specific recognition of NID1 by NKp44Fc chimeric receptor. (A) Concentrated HEK293T-SN was immunoprecipitated with the indicated Fc molecules and analyzed in 7.5% SDS-PAGE under non-reducing conditions; in parallel, concentrated HEK293T-SN was loaded as a positive control. After blotting, the membrane was probed with mouse anti-NID1 mAb followed by HRP-conjugated anti-mouse IgG mAb. One representative experiment of three is shown. (B) Concentrated HEK293T-SN and rNID1 were analyzed in SDS-PAGE on a 7.5% polyacrylamide gel under non-reducing conditions and, after blotting, probed with the indicated Fc molecules or with anti-NID1 mAb, followed by HRP-conjugated anti-human or anti-mouse IgG secondary reagent, respectively. Molecular weight (MW) markers (kDa) are indicated on the right. One representative experiment of five is shown. (C) ELISA plates were coated with rNID1 and incubated with different concentrations of the indicated Fc molecules followed by HRP-conjugated anti-human IgG mAb. Graph represents absorbance at 405 nm after normalization to background (nonspecific binding of the secondary reagent). Data are medians of triplicates ± interquartile range and are the pooled results of three independent experiments. ****p < 0.0001 by two-tailed Mann-Whitney test.
