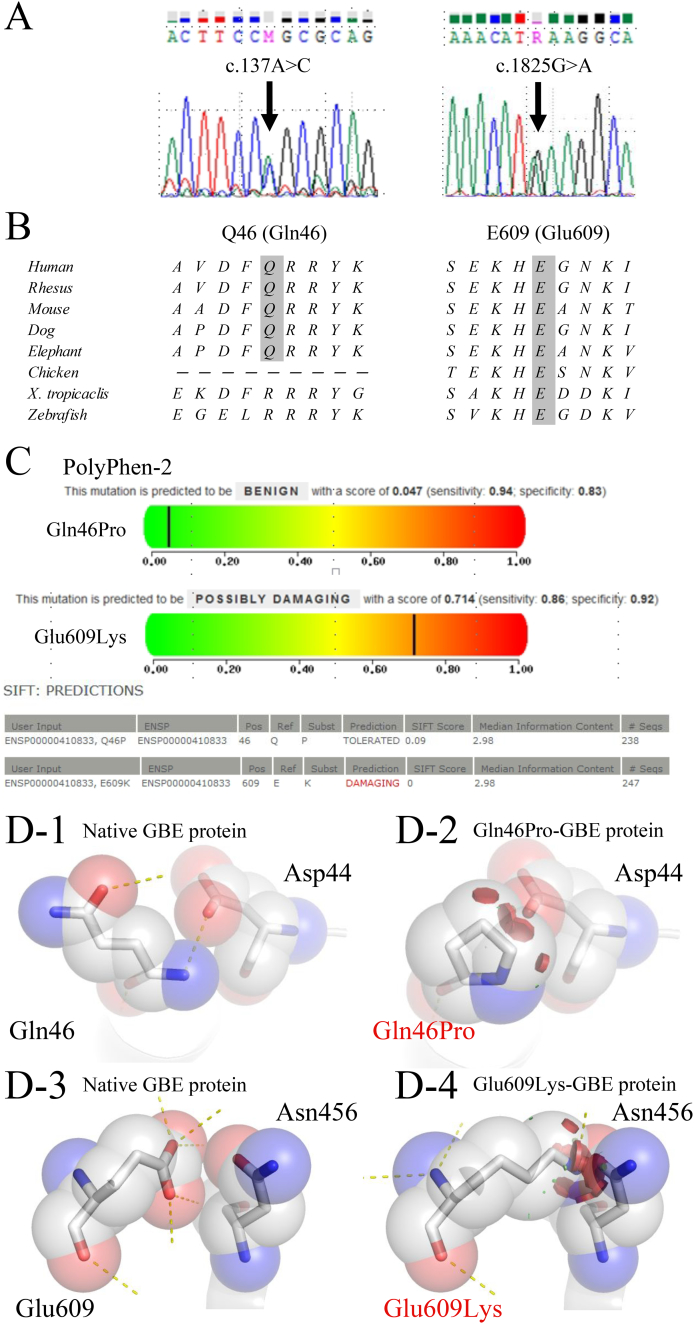
Fig. 3.
GBE1 mutation analysis.
Electropherogram of GBE1 gDNA of our patient, showing heterozygous missense mutations (arrow). (B) Glutamine at position 46 (Gln46) and glutamate at position 609 (Glu609) in Homo sapiens GBE1 are conserved. (C) PolyPhen-2 and SIFT analyses of the p.Gln46Pro and p.Glu609Lys mutations. (D) A structural model of GBE protein generated via PyMOL. Hydrogen bonding is indicated with dashed lines and steric hindrance is indicated with discs. (D-1, D-3) The native GBE structure shows that the Gln46 and Glu609 interact with Asp44 and Asn456, respectively. (D-2, D-4) The modeled structure of the mutant proteins (Gln46Pro-GBE and Glu609Lys-GBE) is speculated to have structural changes caused by steric hindrance with Asp44 and Asn456, respectively.