Abstract
Background
The objective of the study was to estimate the prevalence of pure central neuropathic pain (CNP) and peripheral neuropathic pain (PNP) among patients attending pain clinics in Spain. The study also aimed to analyze factors associated with pain intensity and quality of life (QoL).
Methods
A cross-sectional study was performed including 53 patients with pure CNP and 281 with pure PNP attending in 104 pain clinics in Spain. The revised grading system proposed in 2008 to determine a definite, probable or possible diagnosis of NP was used. Pain features, psychological variables and QoL were assessed. Descriptive, bivariate and multivariate analyses were performed.
Results
The prevalence of pure CNP and PNP amongst neuropathic pain patients was 2.4% (95% CI: 1.7;3.1) and 12.9% (95% CI: 1.5;14.3), respectively. Comorbid anxiety, depression or sleep disorders were high in both groups, but higher in CNP patients (51.1%, 71.4%, respectively). Pain intensity in PNP patients was associated with the presence of depression and sleep disturbances. However, in CNP patients, it was related with pain in the lower limbs. The impairment of QoL was greater in CNP patients than in PNP patients; pain location, presence of depression and sleep disturbance were the factors that most negatively affected QoL. Among PNP patients, women and those with higher pain intensity had worse QoL.
Conclusion
Pain intensity and QoL are affected by different factors in patients suffering from CNP or PNP. Identifying these factors could serve to guide therapeutic strategies and improve the QoL of patients.
Keywords: central neuropathic pain, peripheral neuropathic pain, pain intensity, quality of life
Introduction
Neuropathic pain (NP) is a complex clinical condition of multifactorial etiology that results in considerable societal and economical burden.1 It also affects the quality of life (QoL) of patients and their families.2,3
Despite its importance, few data are available concerning the prevalence of NP in patients attending pain clinics, although some studies carried out in the general population or primary care have reported a significant increase in recent years.4–6
With specific regard to central neuropathic pain (CNP), the lack of information regarding prevalence is notable, most studies focusing on particular CNP conditions, such as spinal cord injury, stroke7,8 or multiple sclerosis,9 where the prevalence ranges from 4.2% in multiple sclerosis to 80% in spinal cord injury.
Regardless of its cause or location, NP is one of the most difficult types of pain to treat,10,11 with some patients showing partial responses to treatment, and patients with similar conditions responding differently to the same pharmacological drug.1 Several reasons have been proposed to explain these results, one of them being its comorbidity with psychiatric disorders.12
The presence of psychiatric disturbances, particularly anxiety and depression, is common in chronic pain patients.13 However, to our knowledge, the comparative analysis of anxiety and depression in a broader range of pure central and peripheral neuropathic chronic pain conditions using data obtained at national level from patients attending pain clinics has not been studied previously, despite the fact that such comorbidity may have a significant effect on QoL and health care utilization.14
In a previous study carried out in several pain clinics in Spain, we reported that around 48% of the patients attending these consultations suffered from NP and 15.7% had a diagnostic label of primary (pure) CNP and/or PNP. Also, our study showed that comorbid depressive or anxiety disorders were present in most patients. Nevertheless, the prevalence of CNP and PNP was not measured and neither was it analyzed in association with mood and anxiety complaints.1
Recently, sleep disorders have been the focus of attention as an outcome of interest in patients with diverse painful conditions. The interaction between poor sleep and pain is of importance because both conditions affect each other. Furthermore, a number of studies15 reveal that these three conditions (depression, chronic pain and sleep disorders) are interconnected.
A review of the literature identifies a lack of epidemiological data analyzing the frequency and characteristics of CNP and PNP on a national scale and studying the effect of anxiety, depression and sleep disturbances on the pain intensity and QoL of these patients. Consequently, we carried out this present study with the aim of estimating the prevalence of pure CNP and PNP among patients attending Spanish pain clinics, using the revised definition and grading system proposed in 2008 by Treede et al.16 As a secondary objective, we analyzed the relationship of anxiety, depression and sleep disturbances with the intensity of pain and QoL in both CNP and PNP patients. We hypothesize that the presence of anxiety, depression and sleep disorders is independently related to more intense pain and a worse QoL in CNP and PNP patients.
Methods
A cross-sectional study was carried out in all pain clinics registered in Spain (N=104). Pain specialists working in this setting who voluntarily agreed to participate were asked to ascertain NP conditions in patients attending their clinics during a single day. The visits could be either initial or follow-up appointments.
A sample of patients receiving treatment in the participating pain clinics during the study day was selected. The inclusion criterion was as follows: adult patients (≥18 years) with NP (according to the definition by the International Association for the Study of Pain) who provided their written informed consent. Patients unable to attend an interview or to complete a questionnaire were excluded. Data were obtained from the patients’ medical records or during the visit.
The sample size was calculated to respond to the main aim of the original study: to determine the prevalence of NP in patients treated in the pain units of Spain. According to the literature,17–19 the population prevalence in Spain and Europe ranges from 6% to 8%. Therefore, an initial estimate of 7% was assumed. With a confidence level of 95%, an accuracy of +/− 0.01 percentage units in a bilateral contrast, and considering a missingness lower than 4%, a total of 2599 patients would be necessary. Of these, ~182 patients diagnosed with NP would be detected (the expected prevalence of 7%). Considering the inclusion of patients from 104 centers, it would be necessary to include 25 patients from each of them in order to obtain information from 2600 patients.
Patients were classified as having definite, probable or possible NP according to the revised definition of NP proposed in 2008.16 The criteria for the classification were as follows: the patient had 1) a distinct neuroanatomically plausible distribution of pain, 2) a history suggestive of a lesion or disease affecting the somatosensory system, 3) a confirmatory test demonstrating the distribution of pain, as part of the neurological examination and 4) a confirmatory test demonstrating the lesion or disease of the somatosensory system. A patient was considered to have “possible neuropathic pain” if they met criteria 1 and 2. The NP was considered “probable” if they additionally met criteria 3 or 4.
The physician also had to give a diagnostic label for the NP condition20 of each patient according to the options presented in Table 1, where an open field labeled as “others” was included within each option to capture rare diagnoses. Some additional information was collected including localization of pain, pain duration (<6 months, 6–12 months, >12 months or number of years) and average pain intensity in the prior 24 h, using a visual analog scale (VAS). Furthermore, information was gathered from the patients’ medical records about whether they suffered any depressive, anxiety, sleep or any other comorbid disorder. A new variable was created indicating the number of comorbid conditions (anxiety, depression or sleep disorder). This variable was set to 0 when the patients did not suffer any comorbidity; 1 when they suffered just one of the three diseases; 2 when the patient suffered two comorbidities (anxiety plus depression or anxiety plus sleep disorder or depression plus sleep disorder); and 3 when the patient suffered from the three pathologies.
Table 1.
Characteristics of the patients with CNP and PNP, N=334
| Characteristic | Central N=53 |
Peripheral N=281 |
p |
|---|---|---|---|
| Sex: females [n/N available (%)] | 33/53 (62.3) | 176/277 (63.5) | 0.860a |
| Current age (years) [mean (SD)] | 59.3 (15.8) | 56.6 (15.5) | 0.243b |
| Ethnicity: | N available=53 | N available=280 | 0.033c |
| Caucasian [n (%)] | 38 (71.7) | 236 (84.3) | |
| Latin American [n (%)] | 15 (28.3) | 40 (14.3) | |
| Arab | 0 (0) | 4 (1.4) | |
| Work status: | N available=53 | N available=280 | 0.306a |
| Retired [n (%)] | 21 (39.6) | 97 (34.6) | |
| Active (employed/housewife) [n (%)] | 12 (22.6) | 96 (34.3) | |
| Temporary/permanent sick leave [n (%)] | 16 (30.2) | 76 (27.1) | |
| Unemployed/student [n (%)] | 4 (7.5) | 11 (3.9) | |
| Type of contact: initial (vs follow-up) [n/N available (%)] | 14/51 (27.5) | 75/273 (27.5) | 0.997a |
| Referral: | N available=52 | N available=279 | 0.001a |
| Traumatology [n (%)] | 11 (23.4) | 70 (30.7) | |
| Neurosurgery/neurology [n (%)] | 24 (51.1) | 50 (21.9) | |
| Primary care [n (%)] | 5 (9.6) | 51 (18.3) | |
| Other (<5% each) [n (%)] | 12 (25.5) | 108 (47.4) | |
| Pain topography: | N available=50 | N available=237 | |
| Head–column–trunk [n (%)] | 32 (64.0) | 68 (28.7) | <0.001a |
| Upper limbs [n (%)] | 20 (40.0) | 68 (28.7) | 0.115a |
| Lower limbs [n (%)] | 25 (50.0) | 102 (43.0) | 0.368a |
| Other [n (%)] | 1 (2.0) | 14 (5.9) | 0.210c |
| Duration of pain symptoms (years) [mean (SD)] | 5.6 (7.3) | 4.1 (5.3) | 0.074d |
| Duration of pain symptoms (categorized): | N available=53 | N available=279 | 0.513a |
| <6 months [n (%)] | 9 (17.0) | 50 (17.9) | |
| 6–12 months [n (%)] | 9 (17.0) | 66 (23.7) | |
| >12 months [n (%)] | 35 (66.0) | 163 (58.4) | |
| Current pain intensity (cm in VAS) [mean (SD)] | 6.5 (1.9) | 6.4 (2.1) | 0.894d |
| Current pain intensity (categorized) | N available=53 | N available=278 | 0.802a |
| Mild pain (VAS score 0–3) [n (%)] | 5 (9.4) | 32 (11.5) | |
| Moderate pain (VAS score 4–5) [n (%)] | 9 (17.0) | 54 (19.4) | |
| Severe pain (VAS score 6–10) [n (%)] | 39 (73.6) | 192 (69.1) | |
| Comorbidities: | N available=49 | N available=236 | |
| Anxiety [n (%)] | 27 (51.1) | 122 (51.7) | 0.664a |
| Depression [n (%)] | 35 (71.4) | 100 (42.4) | <0.001a |
| Sleep disorders [n (%)] | 35 (71.4) | 138 (58.5) | 0.091a |
| Other [n (%)] | 6 (12.2) | 30 (12.7) | 0.929a |
| Numbers of comorbidities: | N available=49 | N available=236 | 0.011a |
| 0 [n (%)] | 1 (2) | 18 (7.6) | |
| 1 [n (%)] | 15 (30.6) | 114 (48.3) | |
| 2e [n (%)] | 17 (34.7) | 66 (28.0) | |
| 3 [n (%)] | 16 (32.7) | 38 (16.1) | |
| Receive adequate treatment: | N available=53 | N available=262 | 0.276a |
| Badly or under-treated [n (%)] | 25 (47.2) | 145 (55.3) | |
| Suitably treated [n (%)] | 28 (52.8) | 117 (44.7) | |
| Current health status (EuroQol-5D) [mean (SD)] | 43.2 (21.3) | 52.3 (21.7) | 0.005d |
| Level of certainty about neuropathic pain: | N available=53 | N available=281 | 0.035a |
| Definite [n (%)] | 43 (81.1) | 208 (74.0) | |
| Probable [n (%)] | 5 (9.4) | 62 (22.1) | |
| Possible [n (%)] | 5 (9.4) | 11 (3.9) |
Notes:
Pearson’s chi-squared test.
Student t-test.
Likelihood ratio.
Mann–Whitney U test.
In the CNP group, 21 (42.9%) patients suffered from anxiety and depression, 20 (40.8%) suffered from anxiety and sleep disorders and 24 (49%) suffered from depression and sleep disorders. In the PNP group, 55 (23.3%) patients suffered from anxiety and depression, 68 (28.8%) suffered from anxiety and sleep disorders and 57 (24.2%) suffered from depression and sleep disorders.
Abbreviations: CNP, central neuropathic pain; PNP, peripheral neuropathic pain; VAS, visual analog scale; EuroQol-5D, EuroQol-5 Dimensions.
Furthermore, the subjective appraisal of the specialist on the adequacy of the treatment received by the patients previous to the contact was recorded based on the clinical state of the patient and on the usual treatment protocols for it. Lastly, the patients were asked to complete the EuroQol-5 Dimensions (EQ-5D) tool21 to provide a measure of health-related QoL. In addition, sociodemographic information including age, sex, ethnicity, work status and the specialty of the referring physician were recorded.
This paper included a subsample composed of patients with pure CNP and PNP. Patients affected by mixed NP were excluded.
The study was performed in accordance with the updated Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Hospital de la Princesa in Madrid before it began. All the patients provided their written informed consent to participate in advance.
Data analysis
For each of the two groups of patients included in the study, a descriptive analysis of the variables was performed with appropriate statistical tools using central tendency and dispersion measurements for the quantitative variables and percentage for the qualitative variables. To analyze the differences between the groups, chi-squared test was used for the qualitative variables, and Student’s t-test or Mann–Whitney U test was used for quantitative variables.
To compare the sociodemographic and clinical characteristics between patients with a definitive diagnosis of NP and patients with probable or possible NP, OR with 95% CI were calculated, and Mantel–Haenszel test, Student’s t-test and the Mann–Whitney U test were used. Lastly, two multiple linear regression models were performed in each group of patients, considering pain intensity and QoL scores as dependent variables and sociodemographic and clinical variables as independent variables.
All the statistical analyses were carried out using the IBM SPSS Statistics 21 package, and the level of significance was set at p<0.05.
Results
Characteristics of the patients with central and peripheral neuropathic pain
Data were collected on 2173 patients attended by 178 pain specialists at 104 pain clinics (81.9% out of 127 pain clinics accredited in Spain [Sociedad Española del Dolor 2011]). A total of 1038 patients were diagnosed with confirmed NP. Of these, this study analyzed the 281 patients diagnosed with pure PNP and 53 with pure CNP. The prevalence of pure CNP in the Spanish pain units was 2.4% (95% CI: 1.77; 3.11) and that of pure PNP was 12.9% (95% CI: 11.50; 14.37). Both groups predominantly consisted of women (62.3% with CNP and 63.5% with PNP) with a slightly higher mean age among those with CNP (59.3 years vs 56.6 years, although the difference was not statistically significant) (Table 1); a high percentage of them were suffering from severe pain (around 70% in both groups). The pain duration was 5.6 years in the patients with CNP and 4.1 years in those with PNP, and it was mainly located in the head–column–trunk (64%) in the patients with CNP and in the lower limbs (43%) in those diagnosed with PNP.
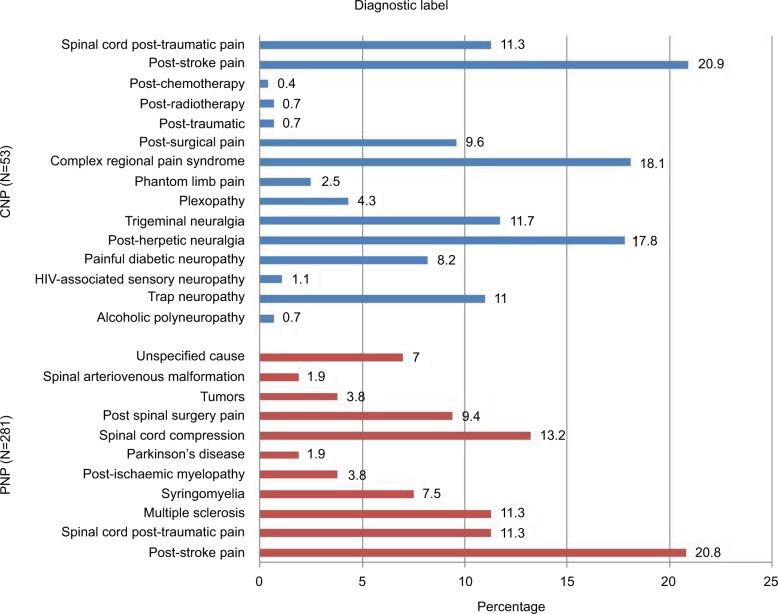
The most common diagnostic label in CNP was post-stroke pain, while complex regional pain syndrome and post-herpetic neuralgia were the most common among the PNP patients (Figure 1).
Figure 1.
Diagnostic labels.
Abbreviations: CNP, central neuropathic pain; PNP, peripheral neuropathic pain.
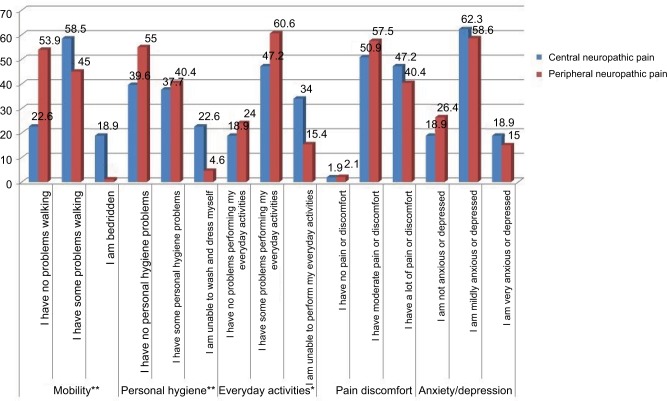
Self-perceived health status, measured with the EQ-5D, was worse in the patients with CNP than in those with PNP (mean EQ-5D VAS score 43.2 and 52.3, respectively); the patients with CNP were more greatly affected in all the dimensions of the EQ-5D (Figure 2).
Figure 2.
Dimensions of the EQ-5D.
Note: *p<0.05; **p<0.01.
Abbreviation: EQ-5D, EuroQol-5 Dimensions.
Of note was that while most of the CNP patients had been derived from Neurosurgery/Neurology department, the PNP patients had been referred by a wide variety of specialists.
Both groups of patients often suffered from comorbid anxiety, sleep disorders and depression, the latter being more common in the CNP patients. In addition, patients in both groups frequently suffered from at least two comorbidities (67.4% in CNP and 44.1% in PNP) (Table 1).
Regarding the level of certainty of the diagnosis, the percentage of patients with definite NP was high and quite similar in both groups (81.1% in CNP and 74% in PNP), although the patients with CNP were more frequently classified with possible NP (9.4% vs 3.9%), while those suffering from PNP were more often diagnosed with probable NP (22.1% vs 9.4%) (Table 1).
Factors associated with the level of certainty of neuropathic pain diagnosis
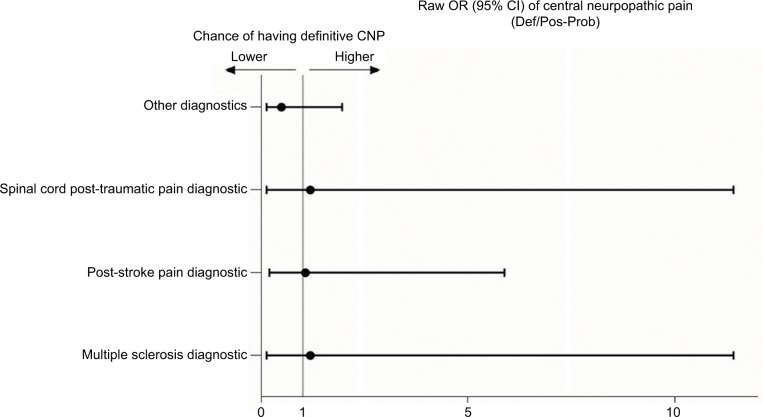
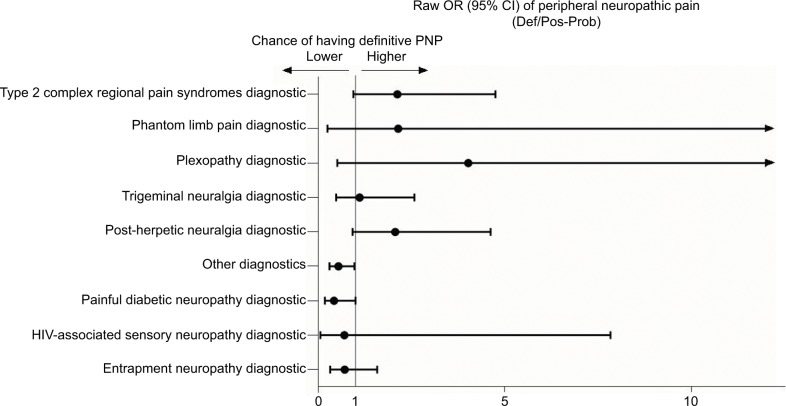
Table 2 and Figures 3 and 4 show the detailed results of the differences between patients classified as having either definite or probable-possible NP. Definite CNP was significantly more likely in patients suffering pain located in the lower limbs. However, in patients with PNP, a definite diagnosis was more frequent when the pain was located in the upper limbs. By contrast, the certainty of the diagnosis was lower among the patients with PNP with pain located in the lower limbs, among those with other less common diagnoses such as post-surgical pain, and in those with diabetic neuropathy, although in this last group the difference was borderline.
Table 2.
Bivariate comparison of patients’ characteristics across levels of certainty of neuropathic pain diagnosis
| Demographics | CNP (N=53)
|
PNP (N=281)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Level of certainty of CNP diagnosis
|
Raw OR (95% CI)a
|
Level of certainty of PNP diagnosis
|
Raw OR (95% CI)a
|
|||||
| Def (N=43)b |
Pos–Pro (N=10)b |
(Def/Pos–Pro) | p | Def (N=208)b |
Pos–Pro (N=73)b |
(Def/Pos–Pro) | p | |
| Sex (being female) [n (%)] | 25 (58.1) | 8 (80.0) | 0.35 (0.07–1.83) | 0.356 | n=204 | n=73 | 0.68 (0.39–1.21) | 0.191 |
| 125 (61.3) | 51 (69.9) | |||||||
| Current age (years) [mean (SD)] | 59.7 (15.9) | 57.5 (16.1) | – | 0.641c | n=207 | 59.5 (14.1) | – | 0.061c |
| 55.6 (15.8) | ||||||||
| Ethnicity (being of Caucasian origin) [n (%)] | 31 (72.1) | 7 (70.0) | 1.11 (0.25–5.00) | 0.895 | n=208 | n=72 | 1.62 (0.81–3.24) | 0.166 |
| 179 (86.1) | 57 (79.2) | |||||||
| Work status (being retired vs. any other) | 17 (39.5) | 4 (40.0) | 0.98 (0.24–4.00) | 0.978 | n=208 | n=72 | 0.99 (0.57–1.75) | 0.987 |
| [n (%)] | 72 (34.6) | 25 (34.7) | ||||||
| Work status (being active vs. any other) | 8 (18.6) | 4 (40.0) | 0.34 (0.08–1.51) | 0.300 | n=208 | n=72 | 0.97 (0.56–1.71) | 0.928 |
| [n (%)] | 71 (34.1) | 25 (34.7) | ||||||
| Administrative data | ||||||||
| Type of contact (initial vs. follow-up) | n=41 | 3 (30.0) | 1.17 (0.26–5.94) | 0.841 | n=202 | n=71 | 0.78 (0.42–1.46) | 0.439 |
| [n initial (%)] | 11 (26.8) | 58 (28.7) | 17 (23.9) | |||||
| Referral: | ||||||||
| From Primary Care vs. other [n Primary | 3 (7.0) | n=9 | 0.26 (0.04–1.87) | 0.430 | n=206 | n=73 | 0.92 (0.47–1.83) | 0.817 |
| Care (%)] | 2 (22.2) | 37 (18.0) | 14 (19.2) | |||||
| From Traumatology vs. other | n=40 | n=7 | 0.0 | 0.404 | n=169 | n=59 | 1.80 (0.90–3.60) | 0.094 |
| [n Traumatology (%)] | 8 (20.0) | 3(42.9) | 33(0.06–1.80) | 57 (33.7) | 13 (22.0) | |||
| From Neurology/Neurosurgery vs. other | n=7 | n=7 | 8.12 (0.89–73.84) | 0.089 | n=169 | n=59 | 1.52 (0.71–3.27) | 0.283 |
| [n Neurology (%)] | 23 (57.5) | 1 (14.3) | 40 (23.7) | 10 (16.9) | ||||
| Clinical data | ||||||||
| Pain topography: head-column-trunk [n (%)] | 26 (65.0) | 6 (60.0) | 1.24 (0.28–5.12) | 0.768 | n=174 | n=63 | 1.25 (0.65–2.41) | 0.500 |
| 52 (29.9) | 16 (25.4) | |||||||
| Pain topography: upper limbs [n (%)] | n=40 | 1 (10.0) | 8.14 (0.92–70.41) | 0.071 | n=174 | n=63 | 2.02 (1.00–4.08) | 0.048 |
| 19 (47.5) | 56 (32.2) | 12 (19.0) | ||||||
| Pain topography: lower limbs [n (%)] | n=40 | 2 (20.0) | 5.41 (1.02–28.79) | 0.034 | n=174 | n=63 | 0.46 (0.26–0.82) | 0.008 |
| 23 (57.5) | 66 (37.9) | 36 (57.1) | ||||||
| Pain topography: other [n (%)] | n=40 | 1 (10.0) | – | – | n=174 | n=63 | 0.90 (0.27–2.98) | 0.862 |
| 0 (0.0) | 10 (4.2) | 4 (6.3) | ||||||
| Duration of pain symptoms (years) | n=32 | n=7 | – | 0.730d | n=157 | n=58 | – | 0.329d |
| [mean (SD)] | 5.4 (7.1) | 6.4 (8.3) | 3.8 (4.8) | 4.78 (6.5) | ||||
| Current pain intensity (cm in VAS) | 6.6 (1.9) | 5.8 (1.9) | – | 0.206c | n=206 | n=72 | – | 0.518d |
| [mean (SD)] | 6.3 (2.1) | 6.5 (2.2) | ||||||
| Comorbid depression [n (%)] | n=41 | n=8 | 0.81 (0.14–4.57) | 0.807 | n=174 | n=62 | 1.23 (0.68–2.22) | 0.497 |
| 29 (70.7) | 6 (75.0) | 76 (43.7) | 24 (38.7) | |||||
| Comorbid anxiety [n (%)] | n=41 | n=8 | 0.35 (0.06–1.94) | 0.396 | n=174 | n=62 | 0.84 (0.47–1.51) | 0.564 |
| 21 (51.2) | 6 (75.0) | 88 (50.6) | 34 (54.8) | |||||
| Comorbid sleep disorders [n (%)] | n=41 | n=8 | 3.10 (0.65–14.73) | 0.299 | n=174 | n=62 | 0.71 (0.39–1.29) | 0.261 |
| 31 (75.6) | 4 (50.0) | 98 (56.3) | 40 (64.5) | |||||
| Other comorbidities [n (%)] | n=41 | n=8 | – | – | n=174 | n=62 | 0.81 (0.35–1.87) | 0.619 |
| 6 (14.6) | 0 (0) | 21 (12.1) | 9 (14.5) | |||||
| Adequate treatment for pain [n (%)] | 24 (55.8) | 4 (40.0) | 1.89 (0.47–7.69) | 0.582 | n=192 | n=70 | 1.41 (0.80–2.46) | 0.232 |
| 90 (46.9) | 27 (38.6) | |||||||
| Current health status (EuroQol-5D) | 44.1 (21.7) | 39.3 (20.0) | – | 0.524c | n=204 | n=71 | – | 0.315d |
| [mean (SD)] | 53.3 (20.7) | 49.8 (24.4) | ||||||
Notes:
OR higher and lower than 1 indicate, respectively, greater and smaller probability of a definite diagnosis of neuropathic pain in the category cited (for example, for females with regard to males).
Percentages are calculated using the available cases for each variable (usually less than the total number of cases, N). Mantel–Haenszel test for qualitative variables.
Student’s t-test.
Mann–Whitney U test.
Abbreviations: CNP, central neuropathic pain; Def, definite; OR, odds ratio; PNP, peripheral neuropathic pain; Pos, possible; Pro, probable; VAS, visual analog scale.
Figure 3.
Raw OR of the diagnostics related to the likelihood of having definite CNP diagnosis.
Note: Other diagnostics includes spinal cord compression, post-surgical pain, tumors, spinal arteriovenous malformation, unspecified cause.
Abbreviations: CNP, central neuropathic pain; OR, odds ratios.
Figure 4.
Raw OR of the diagnostics related to the likelihood of having definite PNP diagnosis.
Note: Other diagnostics includes post-traumatic, post-radiotherapy, and post-chemotherapy.
Abbreviations: PNP, peripheral neuropathic pain; OR, odds ratios.
No differences were observed in the certainty of the diagnosis of any other variable analyzed, neither in the patients with PNP nor CNP, although the patients referred from Neurosurgery/Neurology department were more likely to have a diagnosis of definite CNP than those from other specialties (Table 2).
Factors associated with pain intensity and quality of life
The multivariate analysis of the factors associated with the intensity of the CNP showed that suffering from pain in the lower limbs was related to higher pain intensity. On the contrary, patients attending a follow-up appointment at the pain clinic and those with pain in the upper limbs had lower pain intensity (Table 3).
Table 3.
Multivariate analysis of the factors associated with pain intensity
| Variables | CNP (N=46) | PNP (N=159) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Coefficient (TE) |
t | p-value | 95% CI | Coefficient (TE) |
t | p-value | 95% CI | |
| Constant | 9.64 (1.1) | 8.77 | <0.001 | (7.42; 11.86) | 7.97 (0.8) | 10.32 | <0.001 | (6.45; 9.50) |
| Type of contact | ||||||||
| Follow-up | −1.59 (0.5) | −2.99 | 0.005 | (−2.67; −0.52) | −0.74 (0.4) | −2.03 | 0.045 | (−1.46; −0.02) |
| Initial* | ||||||||
| Current state ofhealth; EQ-5D score | −0.02 (0.1) | −1.55 | 0.130 | (0.01; 0.99) | −0.01 (0.0) | −1.92 | 0.057 | (−0.03;0.00) |
| Pain location upper | ||||||||
| limbs | ||||||||
| Yes | −1.13 (0.5) | −2.32 | 0.025 | (−2.10; −0.15) | These variables were not included in this model | |||
| No* | ||||||||
| Pain location lower limbs | ||||||||
| Yes | 1.09 (0.5) | 2.305 | 0.026 | (0.14; 2.05) | ||||
| No* | ||||||||
| Depression | ||||||||
| Yes | These variables were not included in this model | 0.67 (0.3) | 2.16 | 0.032 | (0.06; 1.27) | |||
| No* | ||||||||
| Sleep disorders | ||||||||
| Yes | 0.83 (0.3) | 2.76 | 0.006 | (0.24; 1.43) | ||||
| No* | ||||||||
| Duration of pain | −0.07(0.0) | −2.45 | 0.014 | (−0.12; −0.01) | ||||
| R2 corrected=0.264 | R2 corrected=0.133 | |||||||
Notes:
Reference category. The self-perceived health status was included in the model as a confounding variable.
Abbreviations: CNP, central neuropathic pain; EQ-5D, EuroQol-5 Dimensions; PNP, peripheral neuropathic pain.
Among the patients with PNP, those also suffering from comorbid depression or sleep disorders were found to be affected by higher pain intensity. Conversely, attending a follow-up appointment at the pain clinic, having a better health status measured with the EQ-5D and longer pain duration were significant correlates of lower pain intensity (Table 3).
In the analysis of self-perceived health status among the patients with CNP, those also suffering from comorbid depression or sleep disorders, or with pain in sites other than the lower limbs presented a worse health status. However, the patients from Latin America, those in active labor and those receiving appropriate treatment had a better health status (Table 4).
Table 4.
Multivariate analysis of the factors associated with the Health Status EQ-5D
| Variables | CNP (N=33)
|
PNP (N=203)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (ET) | t | p-value | 95% CI | Coefficient (ET) | t | p-value | 95% CI | |
| Constant | 49.71 (11.0) | 4.52 | <0.001 | (26.98; 72.44) | 56.87 (10.3) | 5.51 | <0.001 | (36.52; 77.21) |
| Age | These variables were not included in this model | 0.18 (0.1) | 2.06 | 0.041 | (0.01; 0.35) | |||
| Sex | ||||||||
| Woman | −8.07 (2.9) | −2.79 | 0.006 | (−13.77; −2.36) | ||||
| Man* | ||||||||
| Work status | ||||||||
| Active | 23.76 (7.2) | 3.30 | 0.022 | (−27.17; −2.30) | 8.21 (3.0) | 2.69 | 0.008 | (2.20; 14.22) |
| Inactive* | ||||||||
| Ethnicity | These variables were not included in this model | |||||||
| Latin American | 24.96 (5.6) | 4.45 | <0.001 | (13.45; 36.47) | ||||
| Caucasian* | ||||||||
| Depression | ||||||||
| Yes | −14.73 (6.0) | −2.45 | 0.025 | (−25.47; −1.92) | ||||
| No* | ||||||||
| Sleep disorders | ||||||||
| Yes | −13.69 (5.7) | −2.41 | 0.025 | (−25.47; −1.92) | ||||
| No* | ||||||||
| Receiving adequate treatment | ||||||||
| Adequately treated | 11.57 (5.3) | 2.17 | 0.041 | (0.51; 22.62) | ||||
| Badly treated or undertreated* | ||||||||
| Location pain spine | ||||||||
| Yes | −15.38 (5.3) | −2.91 | 0.008 | (−26.33; −4.43) | ||||
| No* | ||||||||
| Pain location upper limbs | ||||||||
| Yes | −12.43 (5.6) | −2.22 | 0.036 | (−23.99; −0.87) | ||||
| No* | ||||||||
| Other locations | ||||||||
| Yes | ||||||||
| No* | −62.05 (16.6) | −3.73 | 0.001 | (−96.46; −27.63) | ||||
| Duration of pain | 0.349 (0.3) | 1.01 | 0.325 | (−0.37; 1.07) | −0.16 (0.3) | −0.62 | 0.537 | (−0.67; 0.35) |
| Intensity of pain (VAS) | These variables were not included in this model | −2.44 (0.6) | −3.89 | <0.001 | (−3.68; −1.12) | |||
| Level of certainty | ||||||||
| Definite | 6.86 (3.1 | (0.84; 12.88) | ||||||
| Probable/possible* | ||||||||
| 2.25 | ||||||||
| 0.026 | ||||||||
| R2 corrected=0.605 | R2 corrected=0.159 | |||||||
Notes:
Reference category. Pain duration was included in the model as a confounding variable.
Abbreviations: CNP, central neuropathic pain; EQ-5D, EuroQol-5 Dimensions; PNP, peripheral neuropathic pain.
Among the patients with PNP, the women and those with greater pain intensity presented a worse self-perceived health status. However, the patients who were older, in active labor or had a definite diagnosis of NP had a better health status (Table 4).
Discussion
The present study is, to our knowledge, the first to analyze the prevalence of pure NP according to its central or peripheral physiopathology.
Also of interest is that the diagnosis of these conditions in this study was based on the judgment of a pain specialist using a widely recognized grading system.
A feature of the results of the study is that the prevalence of PNP was 12.9% and that of CNP was 2.4%, with a high percentage of patients from both groups suffering from a severe intensity of pain (≥6).
In addition, pain duration, symptoms of depression and the impact of the illness on QoL were greater in the CNP group compared to those diagnosed with PNP. As expected, the most common diagnosis in the CNP group was post-stroke pain, while in the PNP group CRPS and post-herpetic neuralgia were the most prevalent.
Several studies carried out in the general population have shown that pain with neuropathic characteristics without a specific location afflicts 7%–8% of people,4 although Breiviket al22 reported a prevalence of 4% in a study carried out in Europe following a different methodology. Furthermore, it has been shown that NP is also a common occurrence in primary care settings, with studies showing wide variations in the prevalence data.23,24
In the same vein, other studies focusing on particular etiological conditions have shown variable results in the prevalence of NP, with a prevalence of 8% in patients with herpes zoster,25 16% in diabetic patients25 and 8%, 10%, 28% and 67% among patients suffering from a stroke, Parkinson’s disease, multiple sclerosis and spinal cord injury, respectively.26–32
In a Spanish study carried out in a primary care setting, the prevalence of pure NP was 11.8 % (95% CI: 10.5; 13.2), but most patients were diagnosed with PNP.24 Also, another study showed that approximately half of all patients referred to pain clinics presented NP. However, this information included patients diagnosed with central, peripheral and mixed NP.1 In our study, patients affected by mixed neuropathic pain were excluded in order to have a more homogeneous group of pure NP patients.
Several reasons have been postulated to explain the variability observed between studies, including the lack of clear diagnostic criteria, particularly for central pain,28 different severities of the diseases of the patients included in the study or the diverse case ascertainment methods used, among others.23
In this study, the pain specialists used the grading system proposed in 2008 to reach their diagnosis and they observed that the diagnostic certainty was high among the patients with both CNP and peripheral NP. In addition, the patients were identified with a probable or possible diagnosis of the disease that would have been excluded if other screening tools such as the DN-4, LANSS or PainDETECT had been used.
Furthermore, the study found that pain intensity in the patients with PNP was associated with the presence of depression and sleep disorders, a relationship not found among the CNP patients, for whom the site of the pain was more important, in particular pain in the lower limbs, which was associated with a greater intensity.
Studies in general practice or specialist settings have suggested that NP is associated with anxiety and depression. Some authors, such as Schaefer et al,13 have found that over 50% of the subjects with NP in the US have some level of anxiety (61.9%) and depression (54.4%), with similar results seen across each of the NP conditions, with more severe pain related to a higher score on scales of anxiety and depression. Pérez et al33 have also shown this association in patients with nociceptive, neuropathic or mixed pain, and de Andrés et al34 have reported that over half of the patients with uncontrolled NP were diagnosed with depression and 43% with anxiety. However, neither of these authors analyzed the frequency and association of these processes in patients with CNP and PNP studied separately. Our study showed that around 50% of the patients with CNP and PNP were suffering from anxiety. By contrast, the frequency of depression was higher in the patients with CNP than among those with PNP, and depression was only associated with the intensity of pain among the PNP patients.
Sleep disorders are common in chronic pain patients, 50%–90% of people with pain reporting sleep disturbances, with insomnia being the most common problem. Schaefer et al13 reported sleep disturbances/insomnia in 42% of NP patients but they did not analyze any information about the frequency of this disorder in CNP and PNP. In our study, 71.4% of the patients with CNP and 58.5% of those diagnosed with PNP presented sleep disorders, although the difference was not significant.
Chronic pain sufferers are known to obtain higher scores in measures of depression. As such, it is not uncommon to find depressed individuals reporting sleep problems.15 This could explain the results observed among the CNP patients in the study, where the frequency of depression was higher and consequently that of sleep disorders was too.
Taken together, these findings imply that all three conditions (depression, chronic pain and insomnia) are interconnected. An interesting critical review of the neurobiological factors involved in the interaction between chronic pain, depression and sleep disruption has recently been published by Boakye et al. It includes an in-depth explanation of the multiple brain mechanisms common to these three conditions and a hypothesis about the complex ways they are associated.15
The intensity of pain in both patient groups (with CNP and with PNP) showed an inverse correlation with patient monitoring in the pain unit. This supports the need for the close control of patients treated in these units, where it seems reasonable to believe that it is not common to use ineffective drugs or the sub-therapeutic doses of the analgesic therapy highlighted in other studies as the main causes of uncontrolled pain.34
The inverse relationship between the duration and intensity of pain observed in the patients with PNP is in agreement with observations in other patients and could be explained by the pain tolerance referred to by some authors.35
Regarding QoL, some research has reported low levels of this outcome measure in patients with NP compared with both the general population36 and patients suffering from other chronic conditions.3 Recently, Pérez et al33 reported that the QoL of patients with NP was significantly impaired, although this study presented no information regarding comparison of HRQL between patients with CNP and PNP. In our study, the QoL of the CNP patients was more affected than that of the PNP patients. The factors having the most negative effect on the QoL of the CNP patients were the location of the pain, the presence of depression and sleep disorders.
Furthermore, among the PNP patients, women and those with a greater intensity of pain reported a worse HRQL. Neither depression nor sleep disorders were associated with the QoL of these patients, findings that disagree with those obtained by Radat et al, who found a lower HRQL among patients with PNP suffering from depression and sleep disorders.12 A possible explanation for these differences is that these authors used the Mini International Neuropsychiatric Interview and the MOS-sleep to assess depression and sleep disorders, while our results are based on the information collected regarding the presence of these processes in the patients’ medical records.
Studies carried out in populations with and without pain have constantly shown that women score lower on QoL scales than men, these differences being explained by different clinical, genetic, environmental and social factors.37 Likewise, in agreement with our findings, other authors have shown that pain intensity has a negative effect on the HRQL of patients. Schaefer et al have reported lower scores in physical and mental health components and in each of the dimensions of the SF-12 among subjects with more severe pain.13 Likewise, Pérez et al have reported a low HRQL in mixed, neuropathic and nociceptive groups using the EQ-5D.33
The certainty of the diagnosis (definite diagnosis vs. probable or possible) was a variable associated with a higher HRQL in the PNP patients. These results could be explained by the same reasons given by Agüera et al,38 who found a higher rate of comorbid pain-mood disorder if the reasons for suffering pain were unknown, and also when a precise diagnosis of the cause of pain was lacking. Marazziti et al39 explained that patients with unexplained pain have a higher likelihood of reporting catastrophic thoughts and they tend to think that the origin of their pain is a mystery; they feel that they have lost control and that their physician does not believe their pain to be real.
Finally, a number of limitations of this research can be cited. NP representation in pain clinics may be overestimated with regard to its prevalence in the general population or other clinical settings. Furthermore, the diagnosis of anxiety and/or depression or sleep disturbances was not tested by a specific medical examination or by a neuropsychological evaluation, which could lead to the frequency of these disorders being underestimated. On the other hand, the fact that the information in the paper was collected in 2010 could be a limitation; however, we believe that the lack of data on the prevalence of PNP, especially CNP obtained at a national level in the pain clinics in Spain, justifies the study. The strengths of the study include the use of the grading system proposed by Treede et al in 2008, which has not been used in other studies of this kind, as well as the involvement of pain specialists with extensive experience in the topic and the use of uniform criteria to diagnose the patients.
Conclusion
This study provides original information about the prevalence of pure CNP in pain units in Spain, and analyzes the differences between these patients and those suffering from PNP. It shows that a high percentage of subjects were suffering from comorbid mood and sleep disorders and that both the intensity of pain and QoL are affected by factors that vary between CNP and PNP patients.
Identifying the peculiarities of the patients affected by these processes could serve as a guideline for strategies for treating these patients and improving their QoL.
Acknowledgments
The authors wish to thank Grünenthal Pharma, S.A., Madrid, Spain, for funding this research. The funding source had no involvement in the conduct of the research and/or preparation of the article.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pérez C, Ribera MV, Gálvez R, et al. High prevalence of confirmed, but also of potential and believed, neuropathic pain in pain clinics. Eur J Pain. 2013;17(3):347–356. doi: 10.1002/j.1532-2149.2012.00204.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. doi: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 3.Doth AH, Hansson PT, Jensen MP, Taylor RS. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain. 2010;149(2):338–344. doi: 10.1016/j.pain.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 4.Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152(12):2836–2843. doi: 10.1016/j.pain.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Shadd JD, Ryan BL, Maddocks HL, McKay SD, Moulin DE. Neuropathic pain in a primary care electronic health record database. Eur J Pain. 2015;19(5):715–721. doi: 10.1002/ejp.594. [DOI] [PubMed] [Google Scholar]
- 7.Watson JC, Sandroni P. Central neuropathic pain syndromes. Mayo Clin Proc. 2016;91(3):372–385. doi: 10.1016/j.mayocp.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Harrison RA, Field TS. Post stroke pain: identification, assessment, and therapy. Cerebrovasc Dis. 2015;39(3–4):190–201. doi: 10.1159/000375397. [DOI] [PubMed] [Google Scholar]
- 9.Heitmann H, Biberacher V, Tiemann L, et al. Prevalence of neuropathic pain in early multiple sclerosis. Mult Scler J. 2016;22(9):1224–1230. doi: 10.1177/1352458515613643. [DOI] [PubMed] [Google Scholar]
- 10.Attal N, Cruccu G, Baron R, et al. European Federation of Neurological Societies EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 11.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Radat F, Margot-Duclot A, Attal N. Psychiatric comorbidities in patients with chronic peripheral neuropathic pain: a multicentre cohort study. Eur J Pain. 2013;17(10):1547–1557. doi: 10.1002/j.1532-2149.2013.00334.x. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer C, Mann R, Sadosky A, et al. Burden of illness associated with peripheral and central neuropathic pain among adults seeking treatment in the United States: a patient-centered evaluation. Pain Med. 2014;15(12):2105–2119. doi: 10.1111/pme.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Korff M, Crane P, Lane M, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113(3):331–339. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Boakye PA, Olechowski C, Rashiq S, et al. A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption. Clin J Pain. 2016;32(4):327–336. doi: 10.1097/AJP.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 16.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 17.Montero Homs J, Gutiérrez-Rivas E, Pardo Fernández J, Navarro Darder C, PREVADOL Epidemiological study of prevalence, incidence and neuropathic pain characterization in neurology units. PREVADOL study. Neurologia. 2005;20(8):385–389. [PubMed] [Google Scholar]
- 18.Montero Homs J, Gutiérrez-Rivas E, Pardo Fernández J, Navarro Darder C, PREVADOL Epidemiological study of prevalence, incidence and neuropathic pain characterization in neurology units. PREVADOL study. Neurologia. 2005;20(8):385–389. Spanish. [PubMed] [Google Scholar]
- 19.Carneado-Ruiz J, Morera-Guitart J, Alfaro-Sáez A, Turpín-Fenoll L, Serna-Candel C, Matías-Guíu J. Neuropathic pain as the reason for visiting Neurology: an analysis of its frequency. Rev Neurol. 2005;41(11):643–648. Spanish [with English abstract] [PubMed] [Google Scholar]
- 20.Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60(11):1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 21.Herdman M, Badia X, Berra S. EuroQol-5D: a simple alternative for measuring health-related quality of life in primary care. Aten Primaria. 2001;28(6):425–430. doi: 10.1016/S0212-6567(01)70406-4. Spanish [with English abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 23.van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Pérez C, Saldaña MT, Navarro A, Vilardaga I, Rejas J. Prevalence and characterization of neuropathic pain in a primary-care setting in Spain: a cross-sectional, multicentre, observational study. Clin Drug Investig. 2009;29(7):441–450. doi: 10.2165/00044011-200929070-00002. [DOI] [PubMed] [Google Scholar]
- 25.Haanpää M, Treede F. Diagnosis and classification of neuropathic pain. IASP Clin Updat. 2010;18(7):1–6. [Google Scholar]
- 26.Schwartzman RJ, Grothusen J, Kiefer TR, Rohr P. Neuropathic central pain: epidemiology, etiology, and treatment options. Arch Neurol. 2001;58(10):1547–1550. doi: 10.1001/archneur.58.10.1547. [DOI] [PubMed] [Google Scholar]
- 27.Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103(3):249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 28.Finnerup NB. A review of central neuropathic pain states. Curr Opin Anaesthesiol. 2008;21(5):586–589. doi: 10.1097/ACO.0b013e32830a4c11. [DOI] [PubMed] [Google Scholar]
- 29.Beiske AG, Loge JH, Rønningen A, Svensson E. Pain in Parkinson’s disease: prevalence and characteristics. Pain. 2009;141(1):173–177. doi: 10.1016/j.pain.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain. 1995;61(2):187–193. doi: 10.1016/0304-3959(94)00144-4. [DOI] [PubMed] [Google Scholar]
- 31.Österberg A, Boivie J, Thuomas KÅ. Central pain in multiple sclerosis – prevalence and clinical characteristics. Eur J Pain. 2005;9(5):531–542. doi: 10.1016/j.ejpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS. Pain and dysesthesia in patients with spinal cord injury: a postal survey. Spinal. 2001;39(5):256–262. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- 33.Pérez C, Margarit C, Sánchez-Magro I, de Antonio A, Villoria J. Chronic pain features relate to quality of life more than physiopathology: a cross-sectional evaluation in pain clinics. Pain Pract. 2017;17(7):866–878. doi: 10.1111/papr.12533. [DOI] [PubMed] [Google Scholar]
- 34.de Andrés J, de la Calle JL, Pérez M, López V. Clinical characteristics, patient-reported outcomes, and previous therapeutic management of patients with uncontrolled neuropathic pain referred to pain clinics. Pain Res Treat. 2014;2014:518716. doi: 10.1155/2014/518716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojeda B, Dueñas M, Salazar A, Mico JA, Torres LM, Failde I. Factors influencing cognitive impairment in neuropathic and musculoskeletal pain and fibromyalgia. Pain Med. 2018;19(3):499–510. doi: 10.1093/pm/pnx024. [DOI] [PubMed] [Google Scholar]
- 36.Meyer-Rosberg K, Burckhardt CS, Huizar K, Kvarnström A, Nordfors LO, Kristofferson A. A comparison of the SF-36 and Nottingham Health Profile in patients with chronic neuropathic pain. Eur J Pain. 2001;5(4):391–403. doi: 10.1053/eujp.2001.0260. [DOI] [PubMed] [Google Scholar]
- 37.Racine M, Dion D, Dupuis G, et al. The Canadian STOP-PAIN Project: the burden of chronic pain-does sex really matter? Clin J Pain. 2014;30(5):443–452. doi: 10.1097/AJP.0b013e3182a0de5e. [DOI] [PubMed] [Google Scholar]
- 38.Agüera L, Failde I, Cervilla JA, Díaz-Fernández P, Mico JA. Medically unexplained pain complaints are associated with underlying unrecognized mood disorders in primary care. BMC Fam Pract. 2010;11:17. doi: 10.1186/1471-2296-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marazziti D, Mungai F, Vivarelli L, Presta S, Dell’Osso B. Pain and psychiatry: a critical analysis and pharmacological review. Clin Pract Epidemiol Ment Health. 2006;2(1):31. doi: 10.1186/1745-0179-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]