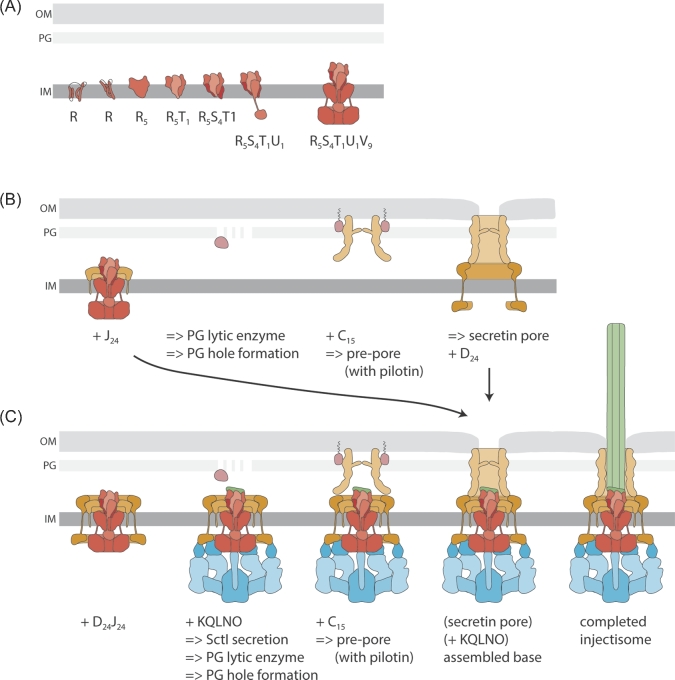
Figure 2.
Assembly of the T3SS injectisome. Protein names are indicated by their last letter, omitting ‘Sct’. The assembly pathways are shown from left to right. A. Assembly of the inner membrane export apparatus starts with membrane-integrated SctR. SctR (and also SctS and SctT, not shown) folds into a conformation that permits helical assembly. Then five SctR, followed by one SctT and four SctS, assemble into a helical assembly, winding up from the membrane. Recruitment of one SctU and then nine SctV finish the export apparatus assembly. B. Outside-in assembly model assuming independent secretin assembly. The export apparatus (the last step in A.) recruits 24 subunits of the inner inner ring protein SctJ. In an independent step, 15 secretin subunits assemble, supported by pilotins, into a pre-pore. PG penetration of the secretin is facilitated by hole-forming PG lytic enzymes. The secretin forms a pore in the outer membrane and recruits the outer inner ring protein SctD. Closure of the outer inner ring is not permitted until the SctJ-export apparatus assembly has been incorporated. C. Inside-out assembly model. The export apparatus (the last step in A.) recruits 24 SctJ and 24 SctD, followed by recruitment of the cytoplasmic components. Type III-dependent secretion of early substrates starts, which locally activates PG lytic enzymes in the periplasm to form a hole for secretin assembly. Just above the inner membrane complex, 15 secretin subunits assemble, supported by pilotins, into a pre-pore. Secretin pore formation and subsequent assembly of the needle filament complete the assembly of the injectisome. Abbreviations: IM, inner membrane; OM, outer membrane; PG, peptidoglycan.