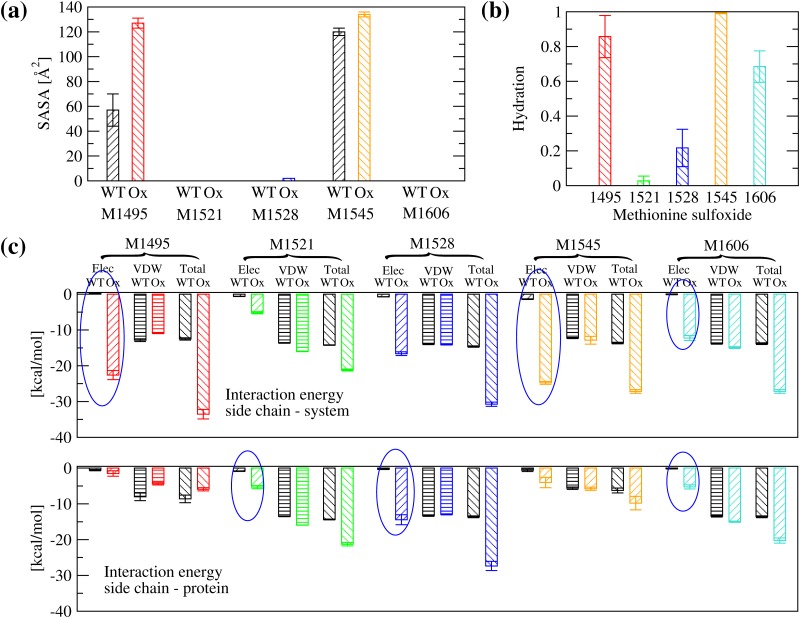
Fig 3. Solvent accessibility and energetic analysis of methionine residues.
(a) SASA of methionine residues in the unoxidized (called here wild-type, WT) and the oxidized (Ox) state. (b) Fraction of frames where a methionine sulfoxide forms at least one hydrogen bond with water molecules. A hydrogen bond was defined using a H…O distance cutoff of 2.7 Å and a O-H…O angle cutoff of 120°. (c) Interaction energy between a methionine side chain and (top) the rest of the system or (bottom) the rest of the protein, respectively. Blue circles highlight whether the change in electrostatic energy upon oxidation is due to the interaction with the solvent or the rest of the protein, or both. The simulations of the oxidized state were performed with all five methionine residues converted to methionine sulfoxide (runs AllMetO_1,2,3 in Table 1) and compared in (a) and (c) to previously published runs with the unoxidized wild-type A2 domain [9]. The represented values are averages over three simulations while error bars denote standard errors of the mean.