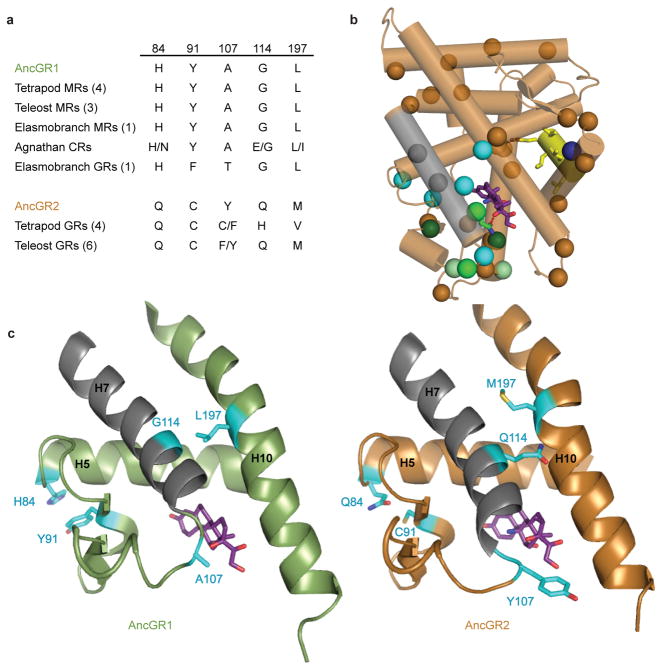
Fig. 2.
Identification of “restrictive” substitutions that impede reversibility. a) Group W residues are conserved in the AncGR1-like state in virtually all extant receptors that retain the ancestral function. b) X-ray crystal structure of AncGR2 (bronze) with dexamethasone (purple). Repositioned Helix 7 is shown in grey. Residues substituted between AncGR1 and AncGR2 are marked with spheres at the α-carbon. Cyan, candidate restrictive substitutions (group W). Sites in groups X, Y, and Z are shown in medium, dark, and light green, respectively. Blue, V234F. c) Ligand pockets of AncGR1 (green, with cortisol) and AncGR2 (bronze, with dexamethasone). Group w residues (cyan) in their ancestral state in AncGR1 are predicted to support the ancestral conformation of helix 7 (grey), but to destabilize that conformation in the derived states of AncGR2.