Abstract
Although plasma biomarkers would facilitate rapid and accurate diagnosis of ischemic stroke for immediate treatment, no such biomarkers have been developed to date. In the present study, we tested our hypothesis that plasma free fatty acids (FFA) are altered at early stages of acute ischemic stroke. Plasma was collected from mice 2 h after permanent middle cerebral artery occlusion (pMCAo) onset, as well as from sham-operated and control animals. After 2 hours, pMCAo significantly changed the plasma FFA profile with the most dramatic 2-to 3-fold relative increase in very long n-3 and n-6 FFA including 20:4n-6, 22:4n-6, 22:5n-6, and 22:6n-3. Changes in plasma FFA profile are consistent with FFA liberation from brain phospholipid hydrolyzed under ischemic insult. These results identify, for the first time, the plasma FFA profile as a potential biomarker for an early ischemic stroke within the therapeutic window for thrombolytic treatment. Further studies are required to confirm its specificity and sensitivity in clinical settings.
Keywords: ischemic stroke, lipids, plasma, free fatty acids, biomarkers
Introduction
Immediate treatment can play a major role in the outcome of ischemic stroke and helps to improve prognosis in many cases (Davis et al., 2006). One of the factors affecting immediate treatment is the rapid and accurate diagnosis of ischemic stroke. In addition to clinical and imaging studies, plasma biomarkers may significantly aid in the early management of ischemic stroke. Importantly, the only FDA-approved therapeutic treatment for ischemic stroke is tissue plasminogen activator administration within 3–4.5 hours of stroke onset. Thus, the ideal biomarker for ischemic stroke would be readily detectible within this window for intravenous thrombolysis (del Zoppo et al., 2009).
To date, the majority of potential stroke biomarkers studied are of a peptide/protein or nucleotide nature and do not meet this requirement (Ahmad et al., 2012). One of the reasons is that these biomarkers are hydrophilic and do not readily cross the blood-brain barrier (BBB). Thus, brain-specific peptides/proteins such as S100b and neuron-specific enolase do not increase in plasma until 10–18 hours post-ischemia and do not correlate with infarct volume prior to 24 hours after injury (Ahmad et al., 2012; Fassbender et al., 1997). Many other brain-specific proteins studied have similar limitations (Ahmad et al., 2012; Fassbender et al., 1997; Kövesdi et al., 2010; Wunderlich et al., 2006), while proteins such as copeptin found elevated under acute stroke conditions are highly non-specific for the brain and may serve as a more general marker of physiologic stress (Katan et al., 2009; Nickel et al., 2012). Another promising approach is utilization of blood gene expression analysis to identify the etiology of acute ischemic stroke (Jauch et al., 2017). However, the timing of RNA expression that requires blood collection 24 hours after stroke onset (Jauch et al., 2017) may add limitations to this approach to meet “the ideal ischemic stroke biomarker” requirements.
However, lipid biomarkers may overcome these limitations. Lipids are hydrophobic and readily cross the BBB, and they account for about 10% of brain wet weight (O’Brien and Sampson, 1965). They have a brain-specific profile with one of the highest arachidonic (20:4n-6), docosahexaenoic (22:6n-3), and adrenic acid (22:5n-6) concentrations esterified in the sn-2 position of glycerophospholipids (Carrié et al., 2000a; Carrié et al., 2000b; Marcheselli et al., 1988). Additionally, alterations in brain lipids develop immediately upon ischemic injury through fatty acid release by a phospholipase A2 family (PLA2) (Bazan, 1970, 1971; Brose et al., 2016; Brose et al., 2011; Golovko and Murphy, 2008; Hamilton et al., 2007). Recently, using a targeted quantification, a brain-specific sphingolipid was identified as a promising biomarker for brain damage 24 h following ischemic stroke onset, demonstrating the feasibility of using lipid biomarkers to diagnose stroke (Sheth et al., 2015).
In the present study, we investigated the use of plasma free fatty acid (FFA) profile as a potential biomarker for early ischemic stroke. Two hours of permanent middle cerebral artery occlusion (pMCAo) significantly changed the plasma FFA profile with the most dramatic 2-to 3-fold relative increase in very long n-3 and n-6 FFA. These results are the first to identify plasma FFA profiles as a potential biomarker for early ischemic stroke within the therapeutic window for thrombolytic treatment. Further studies are required to confirm its specificity and sensitivity in clinical settings.
Methods
Animals
All animal use was approved by the University of North Dakota IACUC (protocol #1503–8). C57BL/6 male mice (22–25g, 8–10 weeks old) were provided standard laboratory chow and water ad libitum.
pMCAo Model and Blood Collection
Animals were fasted overnight before the experiment, and a 2-hour pMCAo was induced using an intraluminal filament as previously described (Jackman et al., 2011). Animals were not given food after the surgery. Briefly, mice were anesthetized with 1.75–2.25% isoflurane, and a standardized microfilament (702345PK5Re, Doccol Corporation, Sharon, MA) was inserted into the internal carotid occlude the origin of middle cerebral artery (MCA). Body temperature was maintained between 36.50C to 37.50C using a heating pad, and was measured with a probe placed rectally. Cerebral blood flow was measured using a Moor Instruments (Axminster, Devon, UK) DRT4 laser Doppler monitor with the sensor placed in the ischemic center (2 mm posterior, 5 mm lateral to the bregma). If blood flow was reduced by less than 80% (20% of the pre-ischemia values), animals were excluded from analysis on the basis of incomplete ischemia. Importantly, the blood flow was recorded from the probe placed on the surface of the scull. Therefore, the meninges blood flow interfered with the cortex flow recording, and this method of monitoring does not allow to detect a 100% blood flow reduction in the ischemic core. A similar surgical procedure was performed in the sham-operated group, except the filament was inserted and immediately withdrawn from the vessel without advancing it to the MCA origin. Two hours after surgery, the animals were anesthetized with ketamine/xylazine (i.p., 100mg and 10 mg per kg, respectively) and blood was collected by a cardiac puncture into heparinized microfuge tubes placed on ice. Control animals were not subjected to surgery prior to blood collection. Plasma was separated by centrifugation and immediately extracted for FFA analysis.
Plasma FFA Extraction and Analysis
Ten μL of plasma was extracted with 90 μL of methanol containing 0.02% BHT (Brose et al., 2013; Brose et al., 2014) and a mixture of internal standards (Cambridge Isotope Laboratories, Tewksbury, MA). For each sample, we used 1 μg of palmitic acid 16:0-13C16 for saturated fatty acids (FA) quantification, 100 ng of 18:1-13C18 for monounsaturated FA, and 100 ng of 20:4n-6-2H8 for polyunsaturated FA (PUFA). After centrifugation, 10 μL of supernatant was injected into the ultra-high-pressure liquid chromatography - mass spectrometry (UPLC-MS) system for analysis.
UPLC-MS analysis was performed as previously described (Brose et al., 2014; Wang et al., 2014). The UPLC system consisted of a Waters ACQUITY UPLC pump with a well-plate autosampler (Waters, Milford, MA) equipped with an ACUITY UPLC HSS T3 column (1.8 μM, 100 A pore diameter, 2.1×150 mm, Waters) and an ACUITY UPLC HSS T3 Vanguard precolumn (1.8 μM, 100 A pore diameter 2.1 × 5 mm, Waters). One microliter of a sample was injected onto the column. The column temperature was 55°C and the autosampler temperature was 8°C.
Solvent A consisted of acetonitrile : water (40 : 60) with 10 μM ammonium acetate and 0.025% acetic acid. Solvent B was acetonitrile : 2-propanol (10 : 90) containing 10 μM ammonium acetate and 0.02% acetic acid. The flow rate was 0.3 mL/min, and the initial %B was 30%. At 0.1 min %B was increased to 54% over 10 min, to 99% over another 10 min, held at 99% for 8 min, and then returned to initial conditions over 0.5 min. The column was equilibrated for 2.5 min between injections.
FA were quantified using a quadrupole time-of-flight mass spectrometer (Q-TOF, Synapt G2-S, Waters) with electrospray ionization in negative ion mode as described previously (Brose et al., 2014; Wang et al., 2014). MassLynx V4.1 software (Waters) was sused for instrument control, acquisition, and sample analysis. FA were quantified against corresponding internal standards using generated standard curves.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad, San Diego; CA). Statistical comparisons were determined using ANOVA with Tukey’s post-hoc test with statistical significance defined as <0.05. All values are expressed as mean ± SD, n=4 animals per group.
Results and Discussion
Although plasma biomarkers would facilitate rapid and accurate diagnosis of early ischemic stroke for immediate treatment, no such biomarkers have been developed to date. Compared to other molecules, FA have a number of advantages as potential stroke biomarkers. They are hydrophobic and readily cross the BBB, have a brain-specific profile, and are released from esterified from within minutes upon ischemia onset.
Previously, using animal models, plasma lipids were analyzed 24 h after transient MCAo (tMCAo) under reperfusion conditions when brain FA may be washed out from the necrotic regions through alterations to the BBB. Under these conditions, 20:4n-6 was increased 2-fold (Rodriguez de Turco et al., 2002), while 22:6n-3 was unaffected. Conflicting with this study, free 20:4n-6 was decreased by 25%, while most n-3 PUFA were increased in plasma in the other report (Paik et al., 2009), although no ANOVA analysis was performed for statistical comparison between the groups in this report. In addition, plasma ceramides were also elevated 24 h after tMCAo (Sheth et al., 2015). In humans, plasma FFA level were positively correlated with the lesion volume when measured 2 days after ischemic stroke event (Chung et al., 2017). However, neither FFA composition nor early time-points were addressed in this study. Importantly, the ideal biomarker for ischemic stroke would be detectible within few hours upon stroke onset to allow intravenous thrombolysis treatment. However, plasma FFA composition at this early time point, as well as under pMCAo when there is a limited reperfusion, has not been previously addressed in animal studies or in clinical settings.
To evaluate plasma FFA profile as a potential biomarker for early ischemic stroke within the therapeutic window for thrombolytic treatment, we analyzed, for the first time, plasma FFA composition 2 h after pMCAo onset (Fig. 1 and 2). The relative concentration (in mole%) of saturated FFA (SFA) was significantly decreased ~35%, while brain-abundant monounsaturated FA (MUFA) and PUFA were significantly increased ~1.6- and ~1.4-fold, respectively, in plasma compared to the control and sham-operated groups. Importantly, there were no differences in plasma FFA between the control and sham-operated animals, indicating that the surgery itself did not have an effect on the observed FFA alterations in plasma. Because the absolute concentration (in nmole/mL) of SFA was unaffected by pMCAo (Fig. 2), the relative decrease in SFA was the result of an increase in plasma-free MUFA and PUFA rather than increased SFA consumption under stroke conditions. The most significantly altered MUFA was 18:1n9 (1.6-fold increase), and among PUFA - 20:4n-6, 22:4n-6, 22:5n-6, and 22:6n-3 (an increase of 1.8- to 2.3-fold compared to the sham and control groups, Fig. 1). This is consistent with immediate phospholipase activation in the ischemic brain (Bazan, 1971; Carrié et al., 2000a) that release brain-abundant FA esterified in phospholipids. Although possible, it is unlikely that plasma FFA are elevated through the liberation from other tissues including adipose tissue. The only significant site of FFA liberation into plasma under physiological and fasting conditions is adipose tissue (Eaton et al., 1969; Mortiaux and Dawson, 1961), however released adipose free fatty acids have a different fatty acid profile with very low mole % of long chain PUFA (Halliwell et al., 1996; Hodson et al., 2008; Malcom et al., 1989), making free fatty acids released from brain distinguishable from this pool in plasma.
Figure 1.
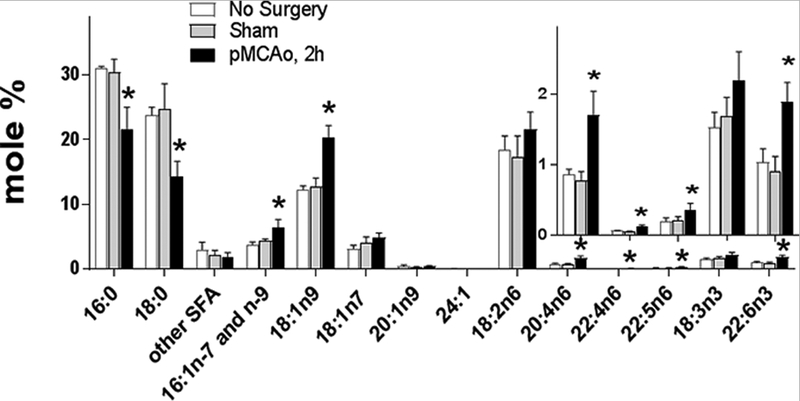
Relative plasma free fatty acid alterations 2 h after pMCAo onset.
Plasma free fatty acids were analyzed two hours after permanent middle cerebral artery occlusion (pMCAo), in sham operated, and control animals using a UPLC-MS method against stable isotope labeled internal standards. Data are mean ± SD (n=4). * - Statistically different (p<0.05) from other groups using ANOVA with Tukey post-hoc test.
Figure 2.
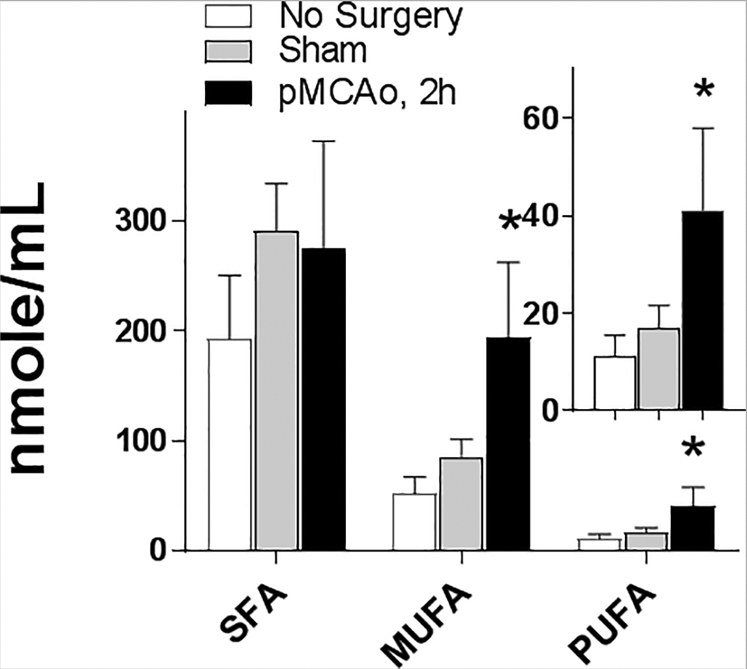
Absolute plasma free fatty acid concentration 2 h after pMCAo onset.
Plasma free fatty acids were quantified two hours after permanent middle cerebral artery occlusion (pMCAo), in sham operated, and control animals using a UPLC-MS method against stable isotope labeled internal standards. SFA: the sum of 14:0, 16:0, 18:0, 20:0, 22:0, and 24:0; MUFA: the sum of 16:1n-7, 16:1n-9, 18:1n-7, 18:1n-9, 20:1n-9, and 24:1; PUFA: the sum of 18:2n-6, 18:3n-3, 20:4n-6, 22:4n-6, 22:5n-6, and 22:6n-3. Data are mean ± SD (n=4). * - Statistically different (p<0.05) from other groups using ANOVA with Tukey post-hoc test.
Additional factors that may potentially limit FFA diagnostic value are human plasma FFA variability/fluctuations and time required for FFA analysis. Although absolute concentration of total FFA is quite variable with ~60% relative standard deviation (Choi et al., 2014; Chung et al., 2017), individual FFA relative concentrations (mole %) are within 10–20% for most plasma FFA (Ågren et al., 1995; Hodson et al., 2008; Jacobsen et al., 1983; Melchert et al., 1987; Umhau et al., 2009; Yli-Jama et al., 2002), indicating that variability is not a limiting factor for using plasma FFA as biomarkers. In addition, long chain free PUFA are not significantly affected by fed/fated state because dietary FA are transported in esterified form, and FFA liberated from adipose tissue do not significantly affect human plasma PUFA (Halliwell et al., 1996). The estimated time for FFA analysis including plasma collection, extraction, and LC-MS analysis, is ~1.5 hour that is within the time required for magnetic resonance imaging, however this time may be decreased in the future with the development of express methods for FFA profiling. However, further validation of plasma FFA profiling specificity for stroke compared to other clinical conditions is needed.
Our results unveil the plasma FFA profile as a potential biomarker for early ischemic stroke within the therapeutic window for thrombolytic treatment. Further studies are required to confirm its specificity and sensitivity in clinical settings.
Acknowledgments:
We would like to thank Mr. Brose for his excellent technical assistance.
Sources of Funding:
This publication was made possible by NIH Grant 5R01 AG042819 05 (MYG), and NIH funded COBRE Mass Spec Core Facility Grant 5P30 GM103329 05 (MYG).
Footnotes
Conflict of Interest:
None
References:
- Ågren JJ, Törmälä ML, Nenonen MT, Hänninen OO, 1995. Fatty acid composition of erythrocyte, platelet, and serum lipids in strict vegans. Lipids 30, 365–369. [DOI] [PubMed] [Google Scholar]
- Ahmad O, Wardlaw J, Whiteley WN, 2012. Correlation of Levels of Neuronal and Glial Markers with Radiological Measures of Infarct Volume in Ischaemic Stroke: A Systematic Review. Cerebrovasc. Dis 33, 47–54. [DOI] [PubMed] [Google Scholar]
- Bazan NG, 1970. Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim. Biophys. Acta 218, 1–10. [DOI] [PubMed] [Google Scholar]
- Bazan NG, 1971. Changes in free fatty acids of brain by drug-induced convulsions, electroshock and anesthesia. J. Neurochem 18, 1379–1385. [DOI] [PubMed] [Google Scholar]
- Brose S, Baker A, Golovko M, 2013. A Fast One-Step Extraction and UPLC–MS/MS Analysis for E2/D2 Series Prostaglandins and Isoprostanes. Lipids 48, 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose SA, Golovko SA, Golovko MY, 2016. Brain 2-Arachidonoylglycerol Levels Are Dramatically and Rapidly Increased Under Acute Ischemia-Injury Which Is Prevented by Microwave Irradiation. Lipids 51, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose SA, Marquardt AL, Golovko MY, 2014. Fatty acid biosynthesis from glutamate and glutamine is specifically induced in neuronal cells under hypoxia. J. Neurochem 129, 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose SA, Thuen BT, Golovko MY, 2011. LC/MS/MS method for analysis of E2 series prostaglandins and isoprostanes. J. Lipid Res 52, 850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrié I, Clément M, de Javel D, Francès H, Bourre J-M, 2000a. Specific phospholipid fatty acid composition of brain regions in mice: effects of n–3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res 41, 465–472. [PubMed] [Google Scholar]
- Carrié I, Clément M, De Javel D, Francès H, Bourre JM, 2000b. Specific phospholipid fatty acid composition of brain regions in mice: Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res 41, 465–472. [PubMed] [Google Scholar]
- Choi JY, Kim JS, Kim JH, Oh K, Koh SB, Seo WK, 2014. High free fatty acid level is associated with recurrent stroke in cardioembolic stroke patients. Neurology 82, 1142–1148. [DOI] [PubMed] [Google Scholar]
- Chung J-W, Seo W-K, Kim G-M, Chung C-S, Lee KH, Bang OY, 2017. Free fatty acid as a determinant of ischemic lesion volume in nonarterial-origin embolic stroke. J. Neurol. Sci 382, 116–121. [DOI] [PubMed] [Google Scholar]
- Davis S, Lees K, Donnan G, 2006. Treating the acute stroke patient as an emergency: current practices and future opportunities. Int. J. Clin. Pract 60, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Saver JL, Jauch EC, Adams HP, 2009. Expansion of the Time Window for Treatment of Acute Ischemic Stroke With Intravenous Tissue Plasminogen Activator. A Science Advisory From the American Heart Association/American Stroke Association 40, 2945–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton RP, Berman M, Steinberg D, 1969. Kinetic studies of plasma free fatty acid and triglyceride metabolism in man. J. Clin. Invest 48, 1560–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Schmidt R, Schreiner A, Fatar M, Mühlhauser F, Daffertshofer M, Hennerici M, 1997. Leakage of brain-originated proteins in peripheral blood: temporal profile and diagnostic value in early ischemic stroke. J. Neurol. Sci 148, 101–105. [DOI] [PubMed] [Google Scholar]
- Golovko MY, Murphy EJ, 2008. Brain prostaglandin formation is increased by α-synuclein gene-ablation during global ischemia. Neurosci. Lett 432, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell KJ, Fielding BA, Samra JS, Humphreys SM, Frayn KN, 1996. Release of individual fatty acids from human adipose tissue in vivo after an overnight fast. J. Lipid Res 37, 1842–1848. [PubMed] [Google Scholar]
- Hamilton JA, Hillard CJ, Spector AA, Watkins PA, 2007. Brain uptake and utilization of fatty acids, lipids and lipoproteins: Application to neurological disorders. J. Mol. Neurosci 33, 2–11. [DOI] [PubMed] [Google Scholar]
- Hodson L, Skeaff CM, Fielding BA, 2008. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res 47, 348–380. [DOI] [PubMed] [Google Scholar]
- Jackman K, Kunz A, Iadecola C, 2011. Modeling focal cerebral ischemia in vivo, Methods Mol. Biol, pp. 195–209. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Trygg K, Hjermann I, Thomassen MS, Real C, Norum KR, 1983. Acyl pattern of adipose tissue triglycerides, plasma free fatty acids, and diet of a group of men participating in a primary coronary prevention program (the Oslo Study). The American Journal of Clinical Nutrition 38, 906–913. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Barreto AD, Broderick JP, Char DM, Cucchiara BL, Devlin TG, Haddock AJ, Hicks WJ, Hiestand BC, Jickling GC, June J, Liebeskind DS, Lowenkopf TJ, Miller JB, O’Neill J, Schoonover TL, Sharp FR, Peacock WF, 2017. Biomarkers of Acute Stroke Etiology (BASE) Study Methodology. Translational Stroke Research 8, 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M, Fluri F, Morgenthaler NG, Schuetz P, Zweifel C, Bingisser R, Müller K, Meckel S, Gass A, Kappos L, Steck AJ, Engelter ST, Müller B, Christ-Crain M, 2009. Copeptin: A novel, independent prognostic marker in patients with ischemic stroke. Ann. Neurol 66, 799–808. [DOI] [PubMed] [Google Scholar]
- Kövesdi E, Lückl J, Bukovics P, Farkas O, Pál J, Czeiter E, Szellár D, Dóczi T, Komoly S, Büki A, 2010. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir. (Wien) 152, 1–17. [DOI] [PubMed] [Google Scholar]
- Malcom GT, Bhattacharyya AK, Velez-Duran M, Guzman MA, Oalmann MC, Strong JP, 1989. Fatty acid composition of adipose tissue in humans: differences between subcutaneous sites. The American Journal of Clinical Nutrition 50, 288–291. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Scott BL, Reddy TS, Bazan NG, Boulton AA, Baker GB, Horrocks LA, 1988. Quantitative analysis of acyl group composition of brain phospholipids, neutral lipids, and free fatty acids, Neuromethods 7 Lipids and Related Compounds. Humana Press, Clifton, NJ, pp. 83–110. [Google Scholar]
- Melchert HU, Limsathayourat N, Mihajlovic H, Eichberg J, Thefeld W, Rottka H, 1987. Fatty acid patterns in triglycerides, diglycerides, free fatty acids, cholesteryl esters and phosphatidylcholine in serum from vegetarians and non-vegetarians. Atherosclerosis 65, 159–166. [DOI] [PubMed] [Google Scholar]
- Mortiaux A, Dawson AM, 1961. Plasma free fatty acid in liver disease. Gut 2, 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel CH, Bingisser R, Morgenthaler NG, 2012. The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Medicine 10, 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JS, Sampson EL, 1965. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J. Lipid Res 6, 537–544. [PubMed] [Google Scholar]
- Paik MJ, Li WY, Ahn YH, Lee PH, Choi S, Kim KR, Kim YM, Bang OY, Lee G, 2009. The free fatty acid metabolome in cerebral ischemia following human mesenchymal stem cell transplantation in rats. Clin. Chim. Acta 402, 25–30. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Belayev L, Liu Y, Busto R, Parkins N, Bazan NG, Ginsberg MD, 2002. Systemic fatty acid responses to transient focal cerebral ischemia: influence of neuroprotectant therapy with human albumin. J. Neurochem 83, 515–524. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Iavarone AT, Liebeskind DS, Won SJ, Swanson RA, 2015. Targeted Lipid Profiling Discovers Plasma Biomarkers of Acute Brain Injury. PLoS ONE 10, e0129735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau JC, Zhou W, Carson RE, Rapoport SI, Polozova A, Demar J, Hussein N, Bhattacharjee AK, Ma K, Esposito G, Majchrzak S, Herscovitch P, Eckelman WC, Kurdziel KA, Salem N, 2009. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J. Lipid Res 50, 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li J, Dasgupta S, Zhang L, Golovko M, Golovko S, Fang J, 2014. Alterations in Membrane Phospholipid Fatty Acids of Gram-Positive Piezotolerant Bacterium Sporosarcina sp. DSK25 in Response to Growth Pressure. Lipids 49, 347–356. [DOI] [PubMed] [Google Scholar]
- Wunderlich MT, Wallesch CW, Goertler M, 2006. Release of glial fibrillary acidic protein is related to the neurovascular status in acute ischemic stroke. Eur. J. Neurol 13, 1118–1123. [DOI] [PubMed] [Google Scholar]
- Yli-Jama P, Meyer HE, Ringstad J, Pedersen JI, 2002. Serum free fatty acid pattern and risk of myocardial infarction: a case-control study. J. Intern. Med 251, 19–28. [DOI] [PubMed] [Google Scholar]


