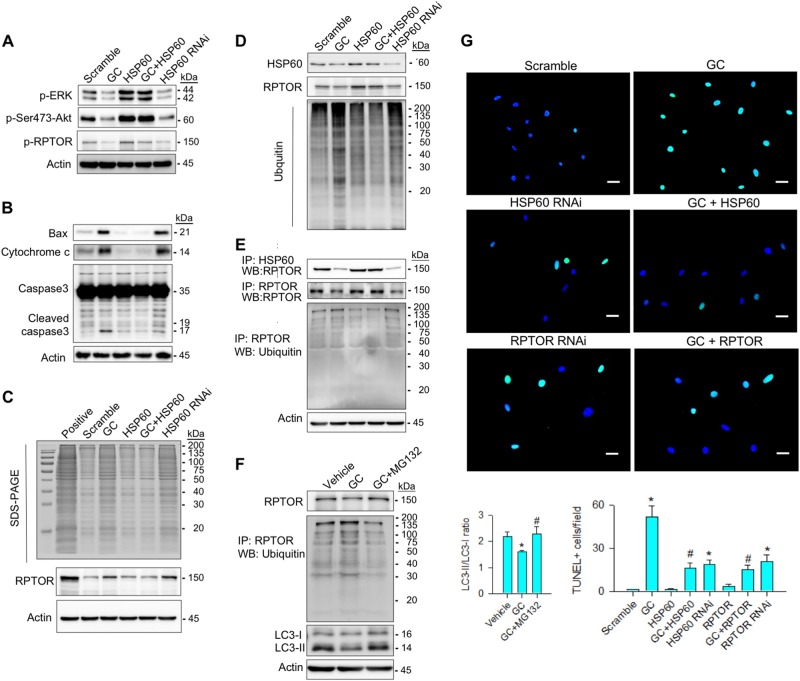
Fig. 4. HSP60 protected RPTOR from the glucocorticoide-phosphorylation, aggregation, and ubiquitination.
a HSP60 restored the glucocorticoid-mediated loss of phosphorylated ERK, phosphorylated Ser473-Akt, and phosphorylated RPTOR abundances. b It attenuated glucocorticoid-enhanced Bax, cytochrome c, and cleaved caspase3 levels. c Forced HSP60 expression alleviated the glucocorticoid elevation of protein aggregation along with decreased RPTOR aggregation. Actin immunoblots indicate equal amount of cell lysates pipetted for protein aggregation assay. d, e HSP60 overexpression reduced the glucocorticoid-upregulated levels of ubiquitinated proteins and RPTOR. Knocking down HSP60 impaired RPTOR phosphorylation but increased RPTOR aggregation and ubiquitination. f MG132 attenuated the glucocorticoid-induced RPTOR ubiquitination and restored RPTOR and LC3-II levels and LC3-II/LC3-I ratio. g Images of TUNEL staining in osteoblasts. HSP60 and RPTOR attenuated glucocorticoid-mediated apoptosis. Scale bar, 85 μm. Experiments results are expressed as mean ± standard error. Asterisks (*) resemble a significant difference (P < 0.05) vs. scramble group, and hashtag (#) indicates a distinguishable difference (P < 0.05) vs. glucocorticoid-treated group