Abstract
Background and purpose
Functional PET/MRI has great potential to improve radiotherapy planning (RTP). However, data integration requires imaging with radiotherapy-specific patient positioning. Here, we investigated the feasibility and image quality of radiotherapy-customized PET/MRI in head-and-neck cancer (HNC) patients using a dedicated hardware setup.
Material and methods
Ten HNC patients were examined with simultaneous PET/MRI before treatment, with radiotherapy and diagnostic scan setup, respectively. We tested feasibility of radiotherapy-specific patient positioning and compared the image quality between both setups by pairwise image analysis of 18F-FDG-PET, T1/T2-weighted and diffusion-weighted MRI. For image quality assessment, similarity measures including average symmetric surface distance (ASSD) of PET and MR-based tumor contours, MR signal-to-noise ratio (SNR) and mean apparent diffusion coefficient (ADC) value were used.
Results
PET/MRI in radiotherapy position was feasible – all patients were successfully examined. ASSD (median/range) of PET and MR contours was 0.6 (0.4–1.2) and 0.9 (0.5–1.3) mm, respectively. For T2-weighted MRI, a reduced SNR of −26.2% (−39.0–−11.7) was observed with radiotherapy setup. No significant difference in mean ADC was found.
Conclusions
Simultaneous PET/MRI in HNC patients using radiotherapy positioning aids is clinically feasible. Though SNR was reduced, the image quality obtained with a radiotherapy setup meets RTP requirements and the data can thus be used for personalized RTP.
Keywords: PET/MRI, Functional imaging, Radiotherapy, Treatment planning, Patient immobilization, Head and neck
Within the scope of radiotherapy (RT) planning, imaging information is primarily used for precise delineation of target volumes and calculation of an optimal radiation dose distribution. In current clinical practice, these two steps of the RT workflow are most commonly based on computed tomography (CT) data, since CT offers high geometric fidelity and provides the electron density information of the tissue [1]. However, in the current era of precision radiation oncology [2], the additional integration of data from different imaging modalities such as (functional) magnetic resonance imaging (MRI) or positron emission tomography (PET) has great potential to improve and individualize RT planning [3], [4], [5]. First, T2- or T1-weighted (contrast-enhanced) MRI shows superior soft tissue contrast as compared to CT. Therefore, it may provide more precise information on tumor localization and spread and thus increase the delineation accuracy of target volumes [6], [7], [8]. In addition, functional information assessed with PET [9], [10], diffusion-weighted (DW) MRI [11], [12], [13] or dynamic contrast-enhanced (DCE) MRI [14], [15], [16] has been associated with RT outcome in different tumor sites including head-and-neck cancer (HNC). Its integration may thus be a promising strategy for adapting RT planning for patients individually [17], [18], [19]. The combination of PET and MRI as a hybrid system now offers spatially and temporally co-registered anatomical and functional image data and may therefore become a key technology for individual therapy adaptation [3], [20], [21], [22], [23].
The integration of combined PET/MRI into RT, however, requires patient examination in RT position for accurate image alignment with the RT planning CT [6], [24], [25], [26], [27]. Especially for HNC, it has been shown that the accuracy of rigid or deformable registration algorithms is strongly improved when patient images are acquired in RT position for both CT and MRI [26], [27]. The adaptation to treatment position, on the other hand, is challenging as it requires RT immobilization equipment to be combined with the MRI hardware, in particular with the radiofrequency coils used for signal reception. For stand-alone MRI systems, dedicated RT coil setups have been presented [28], [29]. However, these setups cannot readily be transferred to a combined PET/MRI system since they do not meet the demands of hardware PET attenuation correction, i.e., foremost, a fixed and reproducible positioning of each hardware device for the usage of predefined attenuation maps [30]. For combined PET/MRI of HNC, an initial RT-specific solution has recently been proposed by Paulus et al. [31]. It comprises a flat table top and MR coil holders for flexible body coils.
In the present study, we upgraded the initial setup with a dedicated add-on, designed and manufactured in-house, for the use of a RT mask fixation system. Besides feasibility assessment of patient imaging in RT treatment position, the aim of the study was to systematically evaluate the image quality of the customized setup in a clinical setting in comparison with a diagnostic setup. Our hypotheses were, that (i) following attenuation correction, good agreement between PET data with RT and diagnostic setup is achievable, that (ii) MR image quality with RT setup is inferior but still sufficient for RT planning applications, and that (iii) potentially reduced MR image quality does not adversely affect the stability of DW-MRI in terms of the mean apparent diffusion coefficient (ADC).
Material and methods
Study design and imaging protocol
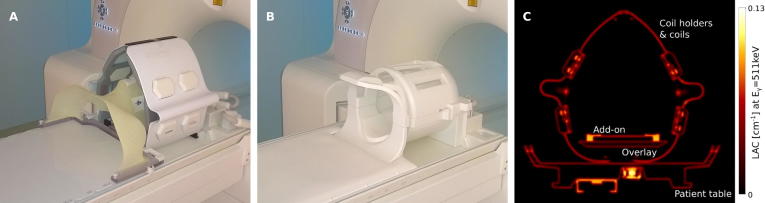
During the pilot phase of a prospective clinical trial (NCT-02666885), ten patients with loco-regionally advanced head-and-neck squamous cell-carcinoma (HNSCC) of the oro- or hypopharynx were examined before the start of multimodal treatment (surgery and adjuvant RT) with simultaneous PET/MRI (Biograph mMR, Siemens Healthcare GmbH, Erlangen, Germany). The imaging protocol included 18F-FDG PET, T2-weighted (T2w) MRI using turbo spin echo (TSE) technique, T1w MRI after contrast agent administration (gadolinium) using volumetric interpolated breath-hold examination (VIBE) technique and DW-MRI (b = 150 and 800 s/mm2). Further protocol details are given in Table 1. PET and MR sequence parameters are listed in Supplementary Tables 1 and 2, respectively. Following intravenous injection of 18F-FDG, two consecutive scans were performed for each patient with RT-specific and diagnostic setup. The RT setup consisted of a flat MR table top and a pair of C-shaped coil holders (Qfix, Avondale, PA, USA) for 6-channel flexible body matrix coils, as introduced in [31]. In addition, an in-house designed add-on was mounted onto the MR table top which allows for patient fixation with a thermoplastic RT mask (ITV, Innsbruck, Austria). The diagnostic setup consisted of the state-of-the-art 16-channel head-and-neck coil of the mMR system. Both hardware setups are depicted in Fig. 1. Hearing protection was ensured using earplugs. Scan limits were infraclavicular level to skull base. For both setups, the first element of the spine array coil was activated to improve signal acquisition in the neck region.
Table 1.
Patient characteristics and imaging protocol.
| Patient | Tumor site | No. of lesion ROIs | Att. corr. FDG-PET | MR-based µ-map | T2w MRI (TSE) | T1w MRI (VIBE) | Diffusion-weighted MRI |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| b = 150 (AP) | b = 150 (PA) | b = 800 (AP) | b = 800 (PA) | ADC (d.c.) | |||||||
| #01 | OP | 2 | ✓ | ✓ | (✓)b | ✓ | ✓ | ✓ | (✓)b | (✓)b | (✓)b |
| #02 | OP/HP/VAL | 3a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| #03 | HP | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| #04 | OP | 2a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| #05 | OP | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| #06 | OP/MC | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| #07 | BT/UV | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| #08 | HP | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| #09 | OP | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| #10 | HP | 1 | ✓ | ✓ | ✓ | ✓ | (✓)c | (✓)c | (✓)c | (✓)c | (✓)c |
Abbreviations: OP – oropharynx; HP – hypopharynx; VAL – vallecula; MC – mouth cavity; BT – base of tongue; UV – uvula; ROI – region of interest; att. corr. – attenuation corrected; TSE – turbo spin echo; VIBE – volume interpolated breath hold; AP – anteroposterior; PA – posteroanterior; d.c. – distortion corrected.
One FDG avid ROI was no RT target; one MR ROI was only weakly PET positive and not considered in PET analysis.
Error in MR sequence parameter settings for scan with diagnostic setup.
Strong geometric distortions in DW-MRI with RT setup.
Fig. 1.
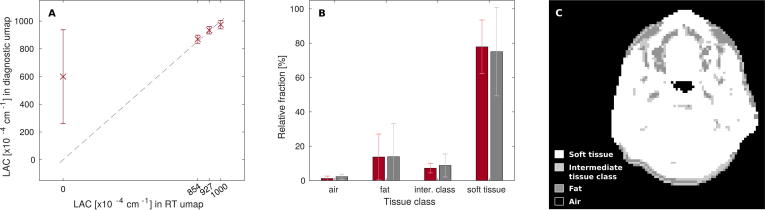
Coil setups for RT specific (A) and diagnostic imaging (B) of the head-and-neck on the Biograph mMR. Setup A consists of a flat table top, C-shaped coil holders and an add-on for patient fixation with a thermoplastic mask. For setup A, a µ-map of the hardware components (C) was generated and used for attenuation correction during PET image reconstruction.
Data processing
Since objects located within the field of view of the PET detector may lead to attenuation and scattering of the PET photons before signal detection, reliable quantification requires data correction. Therefore, attenuation correction was performed during PET data reconstruction using attenuation maps (µ-maps) of both patient and hardware. The individual human µ-map was acquired based on a Dixon fat-water separation technique [32]. For the RT scan, a hardware µ-map was used that was created from CT images of the hardware components by bilinear transformation of CT Hounsfield units into linear attenuation coefficients (LAC) at the characteristic PET photon energy level of Eγ = 511 keV, as described in [31], [33]. For the diagnostic scan, default vendor-supplied attenuation correction of the hardware was applied.
DW-MRI was performed using echo-planar imaging (EPI) technique. Since EPI is sensitive to B0-field inhomogeneities and susceptibility changes especially in the head-and-neck region which can lead to image distortions and signal loss, a method for distortion correction was applied based on repeated data collection with reversed phase-encoding directions (RPED) as described in [34].
Image analysis
The evaluation of PET and MR image quality was performed patient-by-patient by systematic image comparison between RT and diagnostic setup. PET images were compared by estimating the similarity of corresponding regions of interest (ROI). To better account for the difference in positioning between the two examinations, rigid image registration was applied locally with a binary mask using elastix [35]. ROIs were defined in primary tumors and FDG-positive lymph nodes by creating operator-independent 3D threshold contours that comprised voxels with PET activity concentrations greater than or equal to 50% of the local maximum [36]. For pairwise ROI comparison (n = 17), the following similarity measures were calculated in MATLAB R2017b (The MathWorks, Inc., Natick, Massachusetts, United States): Dice similarity index (DSI), relative volume difference (RVD), average symmetric surface distance (ASSD) and Euclidean distance of geometric centers (DOGC).
To evaluate the impact of the RT-tailored coil configuration on MR-based PET attenuation correction, human µ-maps were compared. Rigid registration was applied to the pair of µ-maps in two steps, i.e., for head and neck separately. Nearest neighbor interpolation was chosen for final resampling to preserve discrete µ-map values. For each one of the four tissue classes present in the RT µ-map, its relative fraction and the corresponding mean attenuation coefficient in the reference µ-map were determined.
For MR image quality assessment signal- and contrast-to-noise ratios (SNR, CNR) were calculated. In T2w and distortion corrected DW-MR images, SNR was determined in four anatomical ROIs defined manually in the submandibular glands (left, right) and spinal cord at positions C1-2 and C4-5. In addition, SNR of lesion and CNR of lesion versus adjacent tissue were determined for T2w MRI based on the PET-derived ROIs. Image noise was estimated as the standard deviation (SD) of the signal intensity in a background region [37]. For T1w MRI direct quantitative comparison was not feasible as images were acquired at different times after a single contrast agent administration. To further investigate if MR image quality with RT setup would allow for accurate delineation, RT target structures were contoured manually by a radiation oncologist in training and a board-certified radiologist (KZ, SG) on MR images from both scans using information of T2w and contrast-enhanced T1w MRI. Rigid image registration allowed for the calculation of similarity measures between the ROI pairs (DSI, RVD, ASSD and DOGC).
To assess the stability of DW-MRI, ADC maps were derived from the distortion corrected b-value images. Mean ADC values were compared between RT and diagnostic setups in the lesions based on the PET-derived ROIs. A variability or repeatability coefficient was calculated as the SD of ADC percentage change multiplied by 1.96 [38].
To assess the difference (i) in SNR and CNR in T2w and DW-MRI and (ii) in ADC values between the scan setups, statistical analysis was performed using a Wilcoxon signed rank test (MATLAB R2017b). In either case, a p-value below .05 was considered statistically significant.
Results
All patients were successfully examined with simultaneous 18F-FDG PET, anatomical and DW-MRI in diagnostic as well as in RT setup using a dedicated hardware solution for RT-specific patient positioning. However, one DW-MRI dataset with RT setup presented strong distortion artifacts, probably due to patient swallowing, which could not be corrected using RPED; one DW-MRI dataset with diagnostic setup was incomplete due to wrong protocol settings.
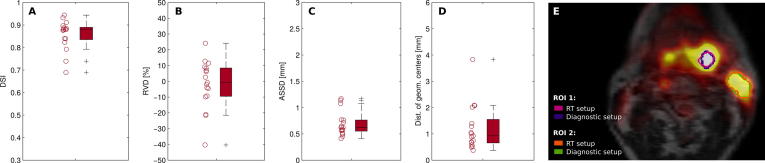
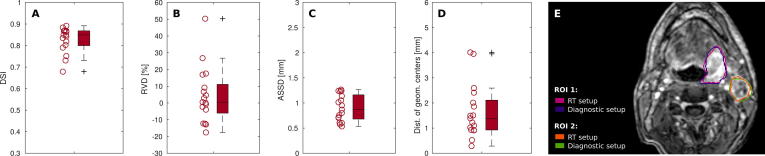
Fig. 2 shows an example for high ROI agreement between RT and diagnostic PET scan. Relative to the measurement with diagnostic setup, the analysis of ROI similarity yielded a cohort median DSI of 0.88 (range: 0.69–0.94) and RVD of −1% (−40–24%). Similarly, median ASSD and DOGC were found to be 0.6 mm (0.4–1.2 mm) and 0.9 mm (0.4–3.8 mm), respectively.
Fig. 2.
Comparison of PET-derived ROIs in FDG-positive tumor and lymph node regions between images acquired with RT and diagnostic scan setup. Boxplots present results of four different similarity measures for all patient ROIs. A-D: Dice similarity index (DSI), relative volume difference (RVD), average symmetric surface distance (ASSD) and the distance of geometric centers (DOGC). E: ROIs in two lesions are shown exemplarily for one patient (#06) on a fused PET/MR image in axial view.
Within regions where the human µ-map with RT setup identified soft tissue, fat, the intermediate class between soft tissue and fat, and air with respective LACs of 1000, 854, 927 and , the following median (range) values were found in the µ-map with diagnostic setup: 989 (898–996), 865 (812–923), 935 (888–976) and 780 (50–993) × 10−4 cm−1, respectively. Mean relative fractions of the four tissue classes in RT and diagnostic µ-map (±SD) were determined as 77.9 ± 15.6/75.1 ± 25.7%, 13.7 ± 13.4/13.9 ± 19.2%, 7.2 ± 2.7/8.8 ± 6.7% and 1.2 ± 1.4/2.3 ± 1.5%, respectively. We refer to Supplementary Fig. 1 for data plots and an exemplary µ-map.
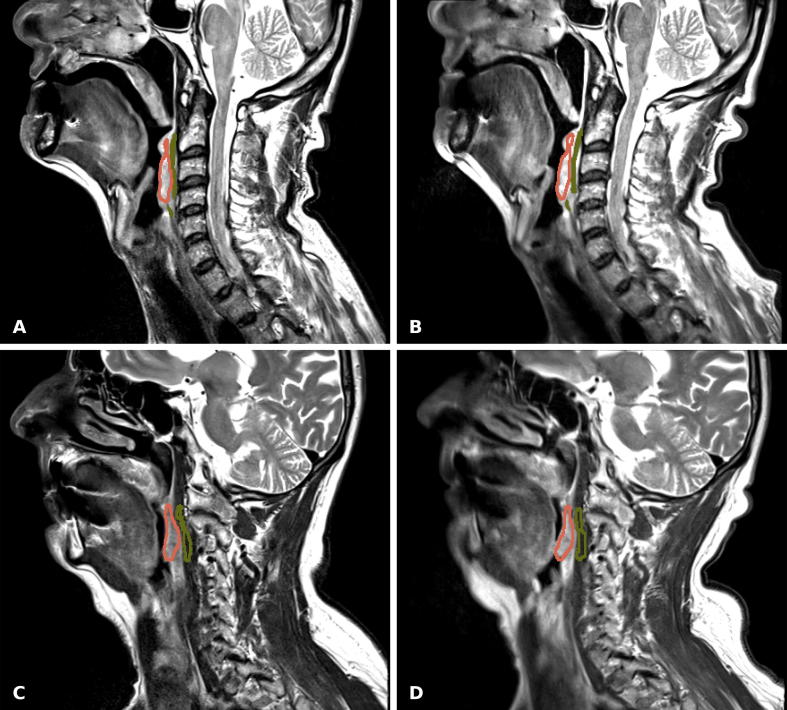
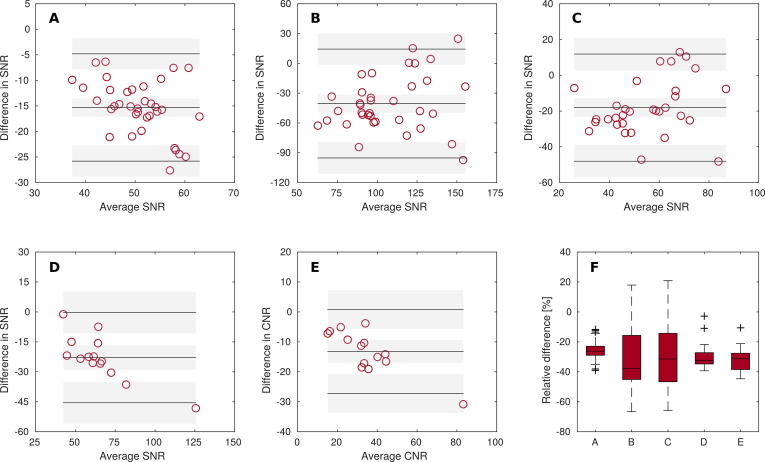
Exemplary images of T2w MRI with RT and diagnostic setup are presented in Fig. 3. ROI-based analysis of T2w and distortion corrected DW-MRI with b-values of 150 and 800 s/mm2 resulted in a cohort median difference in SNR of −26.2% (−39.0–−11.7%), −37.9% (−66.7–17.9%) and −31.4% (−65.9–20.9%), respectively. Similarly, a relative difference of SNR in lesion of −32.2% (−39.3–2.8%) and of CNR in lesion versus adjacent tissue of −31.3% (−44.7–−10.6%) was found between the coil setups for T2w MRI (Fig. 4). Differences in SNR and CNR were found to be significant for both T2w and DW-MRI .
Fig. 3.
T2w MRI using turbo spin echo (TSE) technique of two patients (A, B: #05; C, D: #09) acquired with RT specific (A, C) and diagnostic scan setup (B, D). Note the difference in patient positioning between both scans. ROIs are shown for tumor (red) and adjacent tissue (green). The difference in tumor SNR and CNR in (A) relative to (B) was −21.8% and −21.1%, respectively (good correspondence). Similarly, 30.7% and −31.5% were found for (C) relative to (D) (average correspondence).
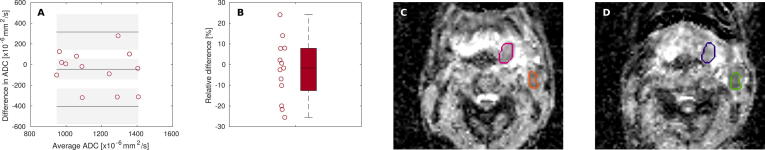
Fig. 4.
Bland Altman plots (A–E) for SNR and CNR for measurements with RT and diagnostic setup. Graphs show difference between two measurements plotted against their average. Solid lines indicate the mean of differences and the limits of agreement (mean ± 1.96 × SD). Corresponding confidence intervals are shown as gray-shaded areas. A–C: SNR in anatomical ROIs for T2w and distortion-corrected DW-MRI with b-values of 150 and 800 s/mm2, respectively. D, E: SNR in lesion and CNR in lesion vs. adjacent tissue for T2w MRI, respectively. F: Boxplot for the relative difference of SNR and CNR between both scan setups for A–E, accordingly.
However, high similarity was found for MR delineated contours. Median DSI and RVD were 0.85 (0.68–0.89) and 0% (−18–50%), respectively. ASSD and DOGC were 0.9 mm (0.5–1.3 mm) and 1.4 mm (0.3–4.0 mm) (Fig. 5).
Fig. 5.
Pairwise comparison of MR delineated target structures between RT and diagnostic scans. Boxplots present results of four different similarity measures for all contours. A–D: Dice similarity index (DSI), relative volume difference (RVD), average symmetric surface distance (ASSD) and the distance of geometric centers (DOGC). E: ROIs in two lesions are shown exemplarily for one patient (#06) on a T1w contrast-enhanced MR image in axial view.
No significant difference was found between ADC values generated with RT and diagnostic setup. For the lesions, a cohort median difference in ADC of −1.7% (−25.5–24.1%) (p = n.s.) was determined (Supplementary Fig. 2). The repeatability coefficient was 17.6%.
Discussion
In personalized RT, treatment adaptation based on PET/MR information could be of particular relevance. However, data integration requires precise alignment with the RT planning CT. Advanced strategies like deformable image registration are available and may yield reasonable results [39], but multimodal alignment is more precise if both examinations are conducted in RT position, especially for head-and-neck [27]. The purpose of this study was to assess feasibility and image quality of a dedicated hardware solution for PET/MRI in treatment position. Image quality assessment, in particular, was based on the pairwise comparison of FDG-PET and MR contours, MRI-derived SNR and mean ADC values between RT and diagnostic setup.
Image analysis of the first ten patients recruited in this clinical trial demonstrated the clinical feasibility of functional PET/MR examination in RT-specific position using a customized hardware setup and dedicated positioning aids – all patients were successfully examined. Although patients reported that mask fixation felt rather tight, the setup was well tolerated and no examination had to be interrupted or aborted.
Certain components of the RT setup used in this study have been presented earlier [31]. Results of phantom-based PET analysis indicated that correct hardware component attenuation correction is feasible. However, the initial setup did not yet allow for patient examination in actual RT position but needed a modified tabletop to allow for the use of head-and-neck immobilization equipment. In the present study, this add-on was designed and a CT-based attenuation map was generated for attenuation correction.
ROI-based analysis showed that PET images acquired with RT and diagnostic setup could be rated as equivalent with regards to target volume definition, as high volume agreement (high DSI/RVD; low ASSD/DOGC) was found. In particular, the cohort maximum ASSD of 1.17 mm was less than the PET voxel size of 2.8 mm. However, ASSD rather represents a low estimate of residual volume mismatch. Besides that, results may be regarded as conservative estimates as registration uncertainty is included. Direct quantitative PET comparison was not practicable due to variations in physiological tracer uptake between consecutive examinations.
In this study, hardware component µ-maps were used in offline PET data reconstruction toolkit RetroRecon. A method for more automated hardware component attenuation correction has recently been proposed [40] and may further simplify the clinical workflow, in particular if the setup is to be extended to other anatomical regions.
A fat-water separating Dixon sequence was used in this study for generation of human µ-maps. As the flexible coils of the RT setup had a greater distance to the head-and-neck, which comes along with lower SNR, the Dixon-based µ-map was verified toward correct tissue segmentation. Very good agreement of LACs between both scan setups was found except for air segmentation. However, this difference is rather negligible since in the head-and-neck region, the fraction of voxels assigned to air is very low (1.2 ± 1.4 vs. 2.3 ± 1.5% for RT and diagnostic setup, respectively). Moreover, the discordance in air detection is likely caused by the variation of air pockets in the oral or pharyngeal region between the two examinations, rather than by deficiencies in the µ-map generation with RT coil setup.
The results are relevant not only for correct PET quantification but also for future RT planning based on PET/MRI as the sole modality, because MR Dixon sequences are an attractive approach to generate substitute or pseudo CTs [41]. For this purpose, correct tissue classification is crucial for dosimetric accuracy, but we do not expect significant differences for pseudo CTs between the setups as for Dixon-based µ-maps only minor differences were observed, as discussed above.
SNR was measured in T2w and DW-MRI. Of note is that the method for noise estimation was chosen for simplicity and its frequent use while potentially more accurate methods exist especially when parallel imaging techniques are used. However, such approaches may require e.g. specific sequence modification for additional acquisition of noise only data without radiofrequency pulse excitation [42], multiple repeated image acquisition for pixel-by-pixel noise SD or repeated acquisition for a noise estimate based on pixel-by-pixel difference [43].
Reduced SNR and CNR were observed as compared to diagnostic imaging. Yet, the image quality seems to be sufficient for RT planning applications as good agreement was found between target structures delineated on MRI. The level of agreement should be assessed against the level of variability of repeated MR delineation since manual delineation is open to both inter- and intra-observer variation [44]. Recently, these two types of variation were quantified for two head-and-neck specialists by a mean DSI of 0.80 and 0.86, respectively [45]. Our results seem to be in the same order (DSI = 0.83 ± 0.06 (mean ± SD)) and thus support the conclusion that MR image quality with RT setup appears suitable for RT planning requirements.
The purpose of a PET/MR examination for RT planning is rather different from a diagnostic one. Besides uniform patient positioning for precise alignment with RT planning CT, isotropic voxel size and geometric accuracy of MRI are essential [4], [20]. In this protocol, isotropic voxel size was realized for T1w MRI and geometric accuracy was assessed for DW-MRI and reported earlier [34]. To improve accuracy of EPI-based DW-MRI by correction for B0-field inhomogeneities and susceptibility induced image distortions different techniques have been proposed [46], [47], [48]. Here, RPED technique was used. The level of geometric accuracy of DW-MRI after RPED correction was in the order of 1 mm [34]. Of note is that the method may correct for geometric distortions but cannot accurately account for signal loss or pile-up.
Comparison of ADC values within FDG-avid tumor and lymph node regions yielded no significant difference between both scan setups indicating that the RT setup does not adversely influence quantification accuracy of DW-MRI. However, deviations in ADC of up to 25.5% and a repeatability coefficient of 17.6% were observed. These values may seem large but correspond to baseline ADC variability in patients with HNSCC. Based on repeated measurements with a one-week interval in 16 patients, Hoang et al. have determined deviations of up to 25% and a repeatability coefficient of 15% [38]. Hence, we consider that the variation in ADC between our two measurements does not necessarily arise from the difference in imaging setup but may rather reflect the uncertainty of EPI-based DW-MRI in head-and-neck.
One question is whether the ADC variability will not compromise clinically relevant information. This especially applies to the measurement of baseline and intratreatment ADC changes to predict outcome or monitor early treatment response [49], [50]. Clinically this is appealing as it would allow to opt for alternative treatment strategies for patients with poor prognosis or non-responders. It is essential, though, that data interpretation takes into account the high intrinsic variability in ADC and yet, relevant ADC information was found e.g. for HNSCC nodal disease where baseline variability was less than intratreatment change [38].
A general advantage of using immobilization equipment during examination is the reduction in bulk motion artifacts in MR images. Artifacts were less pronounced in MR images acquired with RT mask fixation as compared to the diagnostic setup (data not shown). Artifacts due to swallowing, however, cannot entirely be avoided. Beyond that, the RT setup could potentially still be improved. Closer positioning of the coils to the patient would certainly improve the image quality, but reproducible positioning could become more challenging. Besides increasing the number of averages decreasing the resolution, decreasing the acceleration or reducing TE would give a gain in SNR. However, modifications at the expense of longer acquisition time should be balanced carefully against patient comfort as imaging with mask fixation is demanding. We recommend to opt for a total scan time of no longer than 30 min.
Potentially, similar detail to PET/MRI with RT setup in combination with a planning CT could be obtained by combining data from stand-alone MRI in RT position with a planning PET/CT. It may be with the prospect of direct MR planning for head-and-neck in the future that the value of combined PET/MRI with RT setup becomes most pronounced since the number of examinations could be reduced to one.
In conclusion, simultaneous PET/MR examination of HNC patients using RT positioning aids is clinically feasible. Besides good agreement of PET, the proposed setup comes with a compromise in MR image quality in terms of SNR. However, MR delineation accuracy was not adversely affected and ADC measurement with RT setup was found to be stable. The image quality obtained with RT setup therefore meets RT planning requirements and thus allows for precise integration of PET/MRI for future personalized treatment strategies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
Acknowledgements
This study was supported by the Center for Personalized Medicine (ZPM) of the Eberhard Karls University Tübingen; parts of the research leading to these results have received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement n. 335367.
The authors thank Siemens Healthcare GmbH, Erlangen, Germany and Qfix, Avondale, PA, USA for providing PET/MRI devices.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.radonc.2018.04.018.
Appendix A. Supplementary data
Supplementary Fig. 1.
A: data plot shows linear attenuation coefficient (LAC) values (mean ± standard deviation (SD)) in MR-based patient µ-map acquired with diagnostic setup that were determined within the respective tissue class segments in the RT µ-map. B: relative fraction of each tissue class within the RT (red) and the diagnostic µ-map (gray). Error bars are shown in light color, respectively, and represent 1 × SD. C: axial view of MR-based µ-map of one patient (#02, RT setup) with segmented tissue classes.
Supplementary Fig. 2.
A: Bland Altman plot for mean ADC values in PET-derived ROIs measured with RT and diagnostic setup. Graph shows difference between two measurements plotted against their average. Solid lines indicate the mean of differences and the limits of agreement (mean ± 1.96 × SD). Corresponding confidence intervals are shown as gray-shaded areas. B: relative difference of ADC between both scans. C, D: ADC map of one patient (#06, axial slice) acquired with RT and diagnostic setup, respectively. ROI structures in lesions are shown in color. Note the difference in patient positioning between both scans.
Supplementary Fig. 3.
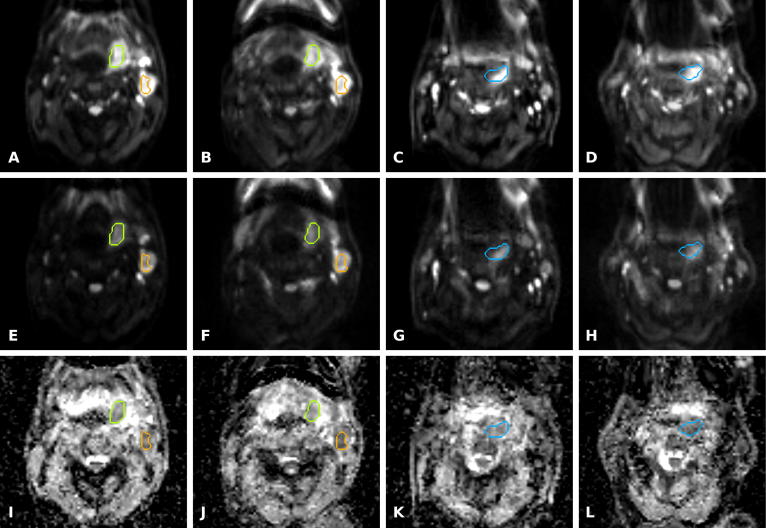
DW-MRI and ADC maps of two patients (#06 and #09) acquired with RT and diagnostic scan setups, respectively. A–D: DW-MRI with b = 150 s/mm2 with RT and diagnostic setup of patient #06 (A, B) and patient #09 (C, D), respectively. E–F: DW-MRI with b = 800 s/mm2 with RT and diagnostic setup of patient #06 (E, F) and #09 (G, H). I–L: Similarly, ADC maps are presented for patient #06 (I, J) and #09 (K, L). PET-derived ROIs are shown for patient #06 (green, orange) and patient #09 (blue) to estimate the difference in ADC. A mean difference in SNR between both scan setups of −24.1% and −16.6% was found for b150 and b800 images for patient #06 (good correspondence). Similarly, a difference of −29.3% and −29.6% was found for patient #09 (average correspondence). The mean difference in ADC was 7.8% and 0.7% for green and orange ROI (good correspondence) and 14% for blue ROI (average correspondence) for patient #06 and #09, respectively.
References
- 1.Pereira G.C., Traughber M., Muzic R.F. The role of imaging in radiation therapy planning: past, present, and future. BioMed Res Int. 2014;2014:1–9. doi: 10.1155/2014/231090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann M., Krause M., Overgaard J. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234–249. doi: 10.1038/nrc.2016.18. [DOI] [PubMed] [Google Scholar]
- 3.Thorwarth D. Functional imaging for radiotherapy treatment planning: current status and future directions—a review. Br J Radiol. 2015;88:20150056. doi: 10.1259/bjr.20150056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Heide U.A., Houweling A.C., Groenendaal G., Beets-Tan R.G.H., Lambin P. Functional MRI for radiotherapy dose painting. Magn Reson Imaging. 2012;30:1216–1223. doi: 10.1016/j.mri.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentzen S.M., Gregoire V. Molecular imaging-based dose painting: a novel paradigm for radiation therapy prescription. Semin Radiat Oncol. 2011;21:101–110. doi: 10.1016/j.semradonc.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt M.A., Payne G.S. Radiotherapy planning using MRI. Phys Med Biol. 2015;60:R323–R361. doi: 10.1088/0031-9155/60/22/R323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagendijk J.J.W., Raaymakers B.W., Van den Berg C.A.T., Moerland M.A., Philippens M.E., van Vulpen M. MR guidance in radiotherapy. Phys Med Biol. 2014;59:R349–R369. doi: 10.1088/0031-9155/59/21/R349. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn F.P., Hullner M., Mader C.E. Contrast-enhanced PET/MR imaging versus contrast-enhanced PET/CT in head and neck cancer: how much MR information is needed? J Nucl Med. 2014;55:551–558. doi: 10.2967/jnumed.113.125443. [DOI] [PubMed] [Google Scholar]
- 9.Min M., Lin P., Lee M.T. Prognostic role of metabolic parameters of 18F-FDG PET-CT scan performed during radiation therapy in locally advanced head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2015;42:1984–1994. doi: 10.1007/s00259-015-3104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welz S., Mönnich D., Pfannenberg C. Prognostic value of dynamic hypoxia PET in head and neck cancer: results from a planned interim analysis of a randomized phase II hypoxia-image guided dose escalation trial. Radiother Oncol. 2017;124:526–532. doi: 10.1016/j.radonc.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Dirix P., Vandecaveye V., De Keyzer F., Stroobants S., Hermans R., Nuyts S. Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with 18F-FDG PET, 18F-Fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J Nucl Med. 2009;50:1020–1027. doi: 10.2967/jnumed.109.062638. [DOI] [PubMed] [Google Scholar]
- 12.Lambrecht M., Van Calster B., Vandecaveye V. Integrating pretreatment diffusion weighted MRI into a multivariable prognostic model for head and neck squamous cell carcinoma. Radiother Oncol. 2014;110:429–434. doi: 10.1016/j.radonc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Noij D.P., Pouwels P.J.W., Ljumanovic R. Predictive value of diffusion-weighted imaging without and with including contrast-enhanced magnetic resonance imaging in image analysis of head and neck squamous cell carcinoma. Eur J Radiol. 2015;84:108–116. doi: 10.1016/j.ejrad.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Wang P., Popovtzer A., Eisbruch A., Cao Y. An approach to identify, from DCE MRI, significant subvolumes of tumors related to outcomes in advanced head-and-neck cancer. Med Phys. 2012;39:5277–5285. doi: 10.1118/1.4737022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla-Dave A., Lee N.Y., Jansen J.F.A. Dynamic contrast-enhanced magnetic resonance imaging as a predictor of outcome in head-and-neck squamous cell carcinoma patients with nodal metastases. Int J Radiat Oncol. 2012;82:1837–1844. doi: 10.1016/j.ijrobp.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng S.-H., Lin C.-Y., Chan S.-C. Dynamic contrast-enhanced MR imaging predicts local control in oropharyngeal or hypopharyngeal squamous cell carcinoma treated with chemoradiotherapy. PLoS One. 2013;8:e72230. doi: 10.1371/journal.pone.0072230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling C.C., Humm J., Larson S. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47:551–560. doi: 10.1016/s0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 18.Bentzen S.M. Theragnostic imaging for radiation oncology: dose-painting by numbers. Lancet Oncol. 2005;6:112–117. doi: 10.1016/S1470-2045(05)01737-7. [DOI] [PubMed] [Google Scholar]
- 19.Thorwarth D., Eschmann S.-M., Paulsen F., Alber M. Hypoxia dose painting by numbers: a planning study. Int J Radiat Oncol. 2007;68:291–300. doi: 10.1016/j.ijrobp.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 20.Thorwarth D., Leibfarth S., Mönnich D. Potential role of PET/MRI in radiotherapy treatment planning. Clin Transl Imaging. 2013;1:45–51. [Google Scholar]
- 21.Leibfarth S., Simoncic U., Mönnich D. Analysis of pairwise correlations in multi-parametric PET/MR data for biological tumor characterization and treatment individualization strategies. Eur J Nucl Med Mol Imaging. 2016;43:1199–1208. doi: 10.1007/s00259-016-3307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen J.H., Nørgaard M., Hansen A.E. Feasibility of multiparametric imaging with PET/MR in head and neck squamous cell carcinoma. J Nucl Med. 2017;58:69–74. doi: 10.2967/jnumed.116.180091. [DOI] [PubMed] [Google Scholar]
- 23.Pinker K., Andrzejewski P., Baltzer P. Multiparametric [18F]Fluorodeoxyglucose/[18F]Fluoromisonidazole positron emission tomography/magnetic resonance imaging of locally advanced cervical cancer for the non-invasive detection of tumor heterogeneity: a pilot study. PLoS One. 2016;11:e0155333. doi: 10.1371/journal.pone.0155333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulson E.S., Crijns S.P.M., Keller B.M. Consensus opinion on MRI simulation for external beam radiation treatment planning. Radiother Oncol. 2016;121:187–192. doi: 10.1016/j.radonc.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Wang K., Mullins B.T., Falchook A.D. Evaluation of PET/MRI for tumor volume delineation for head and neck cancer. Front Oncol. 2017;7 doi: 10.3389/fonc.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanvey S., McJury M., Tho L.M. The influence of MRI scan position on patients with oropharyngeal cancer undergoing radical radiotherapy. Radiat Oncol. 2013;8:129. doi: 10.1186/1748-717X-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortunati V., Verhaart R.F., Verduijn G.M. MRI integration into treatment planning of head and neck tumors: can patient immobilization be avoided? Radiother Oncol. 2015;115:191–194. doi: 10.1016/j.radonc.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Verduijn G.M., Bartels L.W., Raaijmakers C.P.J., Terhaard C.H.J., Pameijer F.A., van den Berg C.A.T. Magnetic resonance imaging protocol optimization for delineation of gross tumor volume in hypopharyngeal and laryngeal tumors. Int J Radiat Oncol. 2009;74:630–636. doi: 10.1016/j.ijrobp.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Hanvey S., Glegg M., Foster J. Magnetic resonance imaging for radiotherapy planning of brain cancer patients using immobilization and surface coils. Phys Med Biol. 2009;54:5381–5394. doi: 10.1088/0031-9155/54/18/002. [DOI] [PubMed] [Google Scholar]
- 30.Paulus D.H., Quick H.H. Hybrid positron emission tomography/magnetic resonance imaging: challenges, methods, and state of the art of hardware component attenuation correction. Invest Radiol. 2016;51:624–634. doi: 10.1097/RLI.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 31.Paulus D.H., Thorwath D., Schmidt H., Quick H.H. Towards integration of PET/MR hybrid imaging into radiation therapy treatment planning. Med Phys. 2014;41:072505. doi: 10.1118/1.4881317. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Moller A., Souvatzoglou M., Delso G. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50:520–526. doi: 10.2967/jnumed.108.054726. [DOI] [PubMed] [Google Scholar]
- 33.Carney J.P.J., Townsend D.W., Rappoport V., Bendriem B. Method for transforming CT images for attenuation correction in PET/CT imaging: transforming CT images for attenuation correction in PET/CT. Med Phys. 2006;33:976–983. doi: 10.1118/1.2174132. [DOI] [PubMed] [Google Scholar]
- 34.Winter R.M., Schmidt H., Leibfarth S. Distortion correction of diffusion-weighted magnetic resonance imaging of the head and neck in radiotherapy position. Acta Oncol. 2017:1–4. doi: 10.1080/0284186X.2017.1377347. [DOI] [PubMed] [Google Scholar]
- 35.Klein S., Staring M., Murphy K., Viergever M.A., Pluim J. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29:196–205. doi: 10.1109/TMI.2009.2035616. [DOI] [PubMed] [Google Scholar]
- 36.Lee J.A. Segmentation of positron emission tomography images: some recommendations for target delineation in radiation oncology. Radiother Oncol. 2010;96:302–307. doi: 10.1016/j.radonc.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Heverhagen J.T. Noise measurement and estimation in MR imaging experiments. Radiology. 2007;245:638–639. doi: 10.1148/radiol.2453062151. [DOI] [PubMed] [Google Scholar]
- 38.Hoang J.K., Choudhury K.R., Chang J., Craciunescu O.I., Yoo D.S., Brizel D.M. Diffusion-weighted imaging for head and neck squamous cell carcinoma: quantifying repeatability to understand early treatment-induced change. Am J Roentgenol. 2014;203:1104–1108. doi: 10.2214/AJR.14.12838. [DOI] [PubMed] [Google Scholar]
- 39.Leibfarth S., Mönnich D., Welz S. A strategy for multimodal deformable image registration to integrate PET/MR into radiotherapy treatment planning. Acta Oncol. 2013;52:1353–1359. doi: 10.3109/0284186X.2013.813964. [DOI] [PubMed] [Google Scholar]
- 40.Paulus D.H., Oehmigen M., Grueneisen J., Umutlu L., Quick H.H. Whole-body hybrid imaging concept for the integration of PET/MR into radiation therapy treatment planning. Phys Med Biol. 2016;61:3504–3520. doi: 10.1088/0031-9155/61/9/3504. [DOI] [PubMed] [Google Scholar]
- 41.Edmund J.M., Nyholm T. A review of substitute CT generation for MRI-only radiation therapy. Radiat Oncol. 2017;12 doi: 10.1186/s13014-016-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kellman P., McVeigh E.R. Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2005;54:1439–1447. doi: 10.1002/mrm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietrich O., Raya J.G., Reeder S.B., Reiser M.F., Schoenberg S.O. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007;26:375–385. doi: 10.1002/jmri.20969. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton C.S., Ebert M.A. Volumetric uncertainty in radiotherapy. Clin Oncol. 2005;17:456–464. doi: 10.1016/j.clon.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Doshi T., Wilson C., Paterson C. Validation of a magnetic resonance imaging-based auto-contouring software tool for gross tumour delineation in head and neck cancer radiotherapy planning. Clin Oncol. 2017;29:60–67. doi: 10.1016/j.clon.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 46.Koyasu S., Iima M., Umeoka S. The clinical utility of reduced-distortion readout-segmented echo-planar imaging in the head and neck region: initial experience. Eur Radiol. 2014;24:3088–3096. doi: 10.1007/s00330-014-3369-5. [DOI] [PubMed] [Google Scholar]
- 47.Windischberger C., Robinson S., Rauscher A., Barth M., Moser E. Robust field map generation using a triple-echo acquisition. J Magn Reson Imaging. 2004;20:730–734. doi: 10.1002/jmri.20158. [DOI] [PubMed] [Google Scholar]
- 48.Gatidis S., Graf H., Weiß J. Diffusion-weighted echo planar MR imaging of the neck at 3 T using integrated shimming: comparison of MR sequence techniques for reducing artifacts caused by magnetic-field inhomogeneities. Magn Reson Mater Phys Biol Med. 2017;30:57–63. doi: 10.1007/s10334-016-0582-z. [DOI] [PubMed] [Google Scholar]
- 49.Vandecaveye V., Dirix P., De Keyzer F. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for head and neck squamous cell carcinoma. Eur Radiol. 2010;20:1703–1714. doi: 10.1007/s00330-010-1734-6. [DOI] [PubMed] [Google Scholar]
- 50.King A.D., Chow K.-K., Yu K.-H. Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology. 2013;266:531–538. doi: 10.1148/radiol.12120167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.