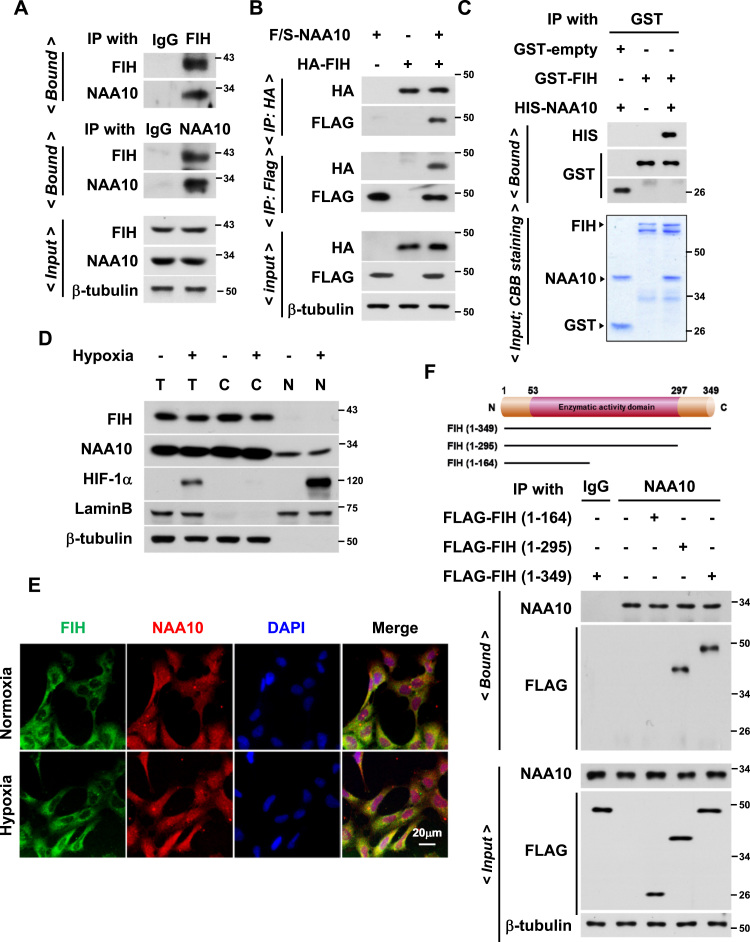
Fig. 1.
FIH and NAA10 coexist in the cytoplasm and interact with each other. (A) Proteins in HEK293 cell lysates were immunoprecipitated by anti-FIH or anti-NAA10 antibody and immunoblotted using the indicated antibodies. (B) HEK293 cells were transfected with HA-FIH (1 μg/ 60-mm dish) and/or FLAG/SBP-NAA10 (1 μg), and the lysates were subjected to immunoprecipitation using HA or FLAG affinity beads. (C) Recombinant proteins GST-FIH (0.5 μg) and His-NAA10 (0.5 μg) were co-incubated in 20 μl of a binding buffer for 1 h. The mixture was pulled down using glutathione affinity beads and immunoblotted (Top). Protein loading was verified by staining gels with Coomassie brilliant blue (Bottom) (D) HEK293 cells were exposed to normoxia (-) or hypoxia (+) for 24 h. Total lysate (T) was fractionated to cytosolic (C) and nuclear (N) components and the samples were immunoblotted. (E) HEK293 cells were cultured in normoxia or hypoxia for 24 h and subjected to immunofluorescence analysis using anti-FIH and anti-NAA10 antibodies. FIH and NAA10 were visualized with Alexa Fluor 488 (green) and Alexa Fluor 594 (red) conjugated secondary antibodies, respectively. Nuclei were stained with DAPI (blue). (F) Schematic diagram of FIH fragments (top). Each FIH fragment was expressed in HEK293 cells, and the cell lysates were subjected to immunoprecipitation and immunoblotting (bottom).