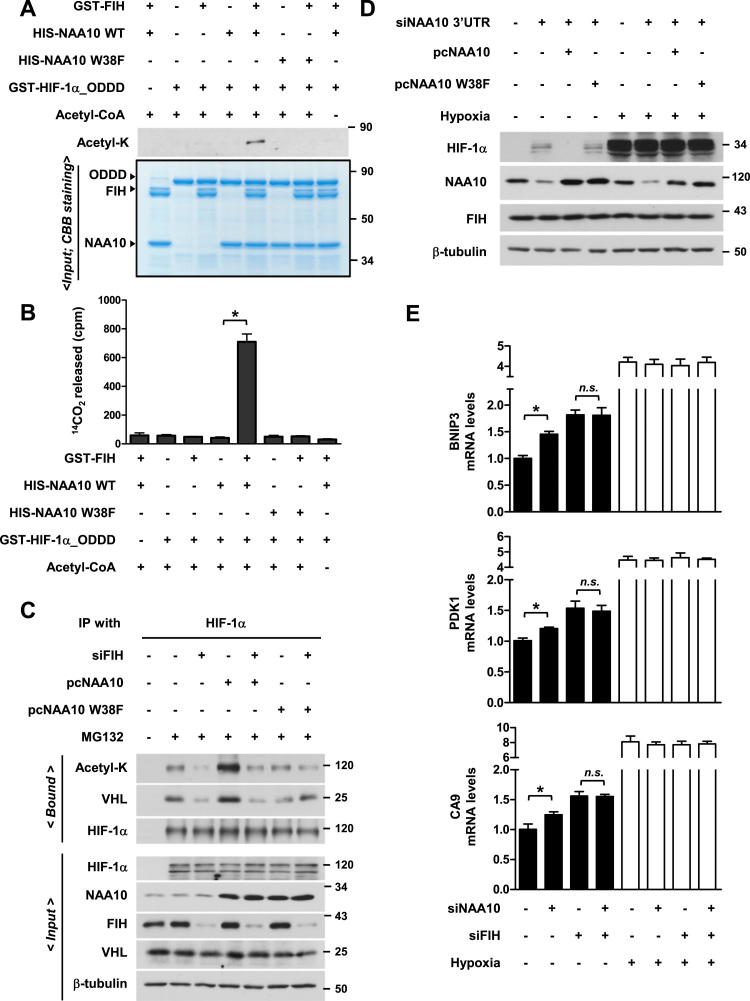
Fig. 4.
W38-hydroxylation enables NAA10 to acquire a lysyl acetyltransferase activity for HIF-1α. (A) In vitro acetylation assay. Firstly, recombinant GST-FIH and His-NAA10 (or W38F) were reacted in an in vitro hydroxylation buffer. Then, GST-HIF-1α ODDD was added as indicated, and further reacted with or without acetyl CoA. Acetylated HIF-1α was detected with anti-acetyl lysine antibody (Top). Protein loading was confirmed by staining gels with Coomassie brilliant blue (Bottom) (B) In vitro acetylation assay using radio isotope. Recombinant proteins were mixed as described in Fig. 4A, and reacted with 200 μM 14C-labeled acetyl CoA and 200 μM cold one. Acetylated HIF-1α was analyzed by scintillation counter. (C) HEK293 cells were co-transfected with the NAA10 (or W38F) plasmid and FIH siRNA (or control siRNA). After treated with 10 μM MG132 for 6 h, cells were subjected to immunoprecipitation with anti-HIF-1α antibody and immunoblotting with the indicated antibodies. (D) HEK293 cells were transfected with a siRNA targeting 3′UTR of NAA10 and/or the NAA10 (or W38F) plasmid. After incubated under normoxia or hypoxia (0.5% O2) for 6 h, cells were subjected to immunoblotting. (E) The mRNA levels of BNIP3, PDK1, and CA9 were quantified by RT-qPCR (n = 3). HEK293 cells which had been transfected with NAA10 and/or FIH siRNAs were incubated under hypoxia (0.5% O2) for 24 h.