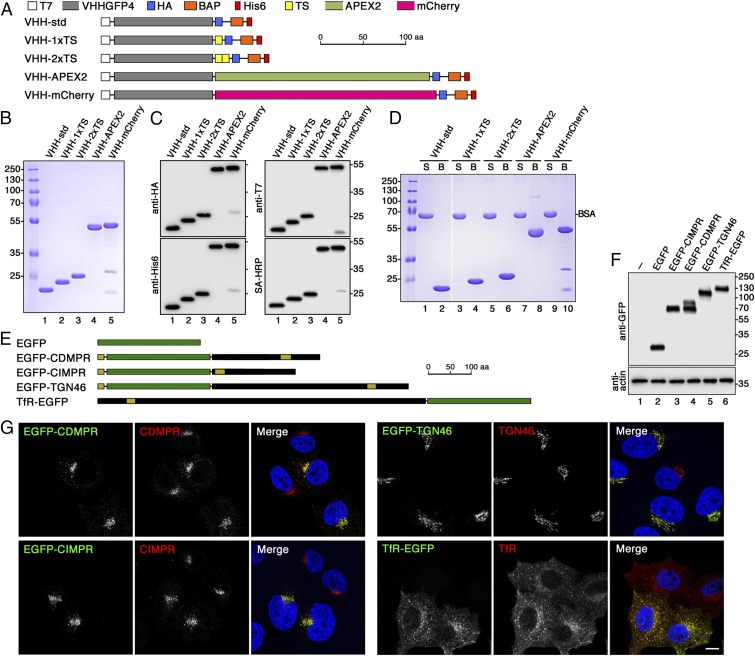
Fig. 1.
Design and production of derivatized nanobodies to track EGFP-tagged surface proteins. (A) Schematic representation of the derivatized nanobodies. The standard nanobody (VHH-std) consists of the GFP-specific VHH domain, T7 and HA epitope tags, a BAP, and a hexahistidine (His6) purification tag. Other nanobodies in addition contain one or two TSs, APEX2, and/or mCherry. Scale bar is in amino acids (aa). (B) Bacterially expressed and purified nanobodies (30 µg) were analyzed by SDS-gel electrophoresis and Coomassie stained. Marker proteins with molecular mass in kiloDaltons are shown on the left. (C) Immunoblot analysis of nanobodies (10 µg) with antibodies against the T7, HA, or His6 epitopes, or with streptavidin-HRP (SA-HRP). (D) The extent of biotinylation was assessed by mixing the nanobodies 1:1 with BSA, incubating the mixture with streptavidin-agarose, pelleting and washing the beads, and analyzing equal fractions of supernatant (S) and material bound to the beads (B) by gel electrophoresis and Coomassie staining. Complete recovery in the bound fraction indicates complete biotinylation of the nanobodies. Recovery of both VHH-mCherry fragments with the beads suggests degradation during sample preparation for SDS-gel electrophoresis. The white line between lanes 2 and 3 indicates deletion of two unrelated lanes. (E) Schematic representation of EGFP (in green) and EGFP fusion proteins. Sequences derived from receptor proteins are shown in black with N-terminal signal peptides and internal transmembrane segments in yellow. EGFP was fused to full-length CDMPR, TGN46, and TfR, and to the transmembrane segment and cytoplasmic sequence of CIMPR. Scale bar in amino acids. (F) HeLa cells stably expressing EGFP-tagged reporter proteins were analyzed by SDS-gel electrophoresis and immunoblotting using anti-GFP antibodies. The positions of size markers with molecular mass in kiloDaltons are indicated. (G) HeLa cells stably expressing the EGFP-tagged reporter proteins were mixed with parental HeLa cells and stained with antibodies targeting the respective endogenous proteins. Except for CIMPR, the antibodies recognize both the endogenous and the EGFP-tagged proteins. The distribution patterns were not altered by expression of the EGFP reporters. Nuclei were stained with DAPI (blue). (Scale bar, 10 µm.)