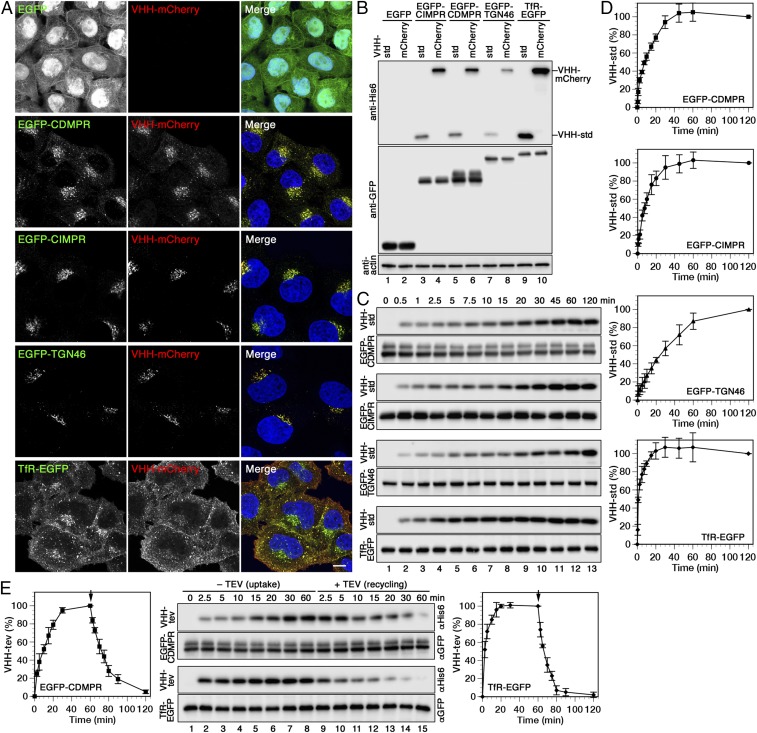
Fig. 2.
Uptake, intracellular localization, and recycling of nanobodies by different EGFP-reporters. (A) Cells expressing cytosolic EGFP or the indicated EGFP fusion proteins were incubated for 1 h at 37 °C with 5 µg/mL VHH-mCherry, fixed, and imaged by fluorescence microscopy. (Scale bar, 10 µm.) (B) The same cell lines were incubated for 1 h at 37 °C with 2 µg/mL VHH-std or 5 µg/mL VHH-mCherry (∼0.1 µM), washed, lysed, and analyzed by immunoblotting for the nanobodies and the EGFP reporters. (The intensity difference between VHH-std and VHH-mCherry is most likely due to better transfer or retention of the larger protein to the blotting membrane.) (C) Kinetics of nanobody uptake were analyzed by incubating the cells at 37 °C for up to 2 h with 2 µg/mL VHH-std. At different times, the cells were lysed, and immunoblotted for cell-associated nanobody (anti-His6) and the EGFP fusion proteins (anti-GFP). (D) Quantitations of nanobody uptake (mean and SD of three independent experiments) are shown in percent of the 120-min values. (E) To determine nanobody uptake and recycling kinetics, cells expressing EGFP-CDMPR or TfR-EGFP were incubated at 37 °C for up to 60 min with 2 µg/mL VHH-tev. Then, cells were washed and further incubated for up to 60 min with 100 µg/mL (2 µM) MBP-TEV protease to remove the hexahistidine tag of nanobody reappearing at the cell surface. Uptake of intact VHH-tev and subsequent TEV cleavage was monitored by immunoblot analysis using anti-His6 antibody, and quantified in percent of the value after 60 min (mean and SD of three independent experiments).