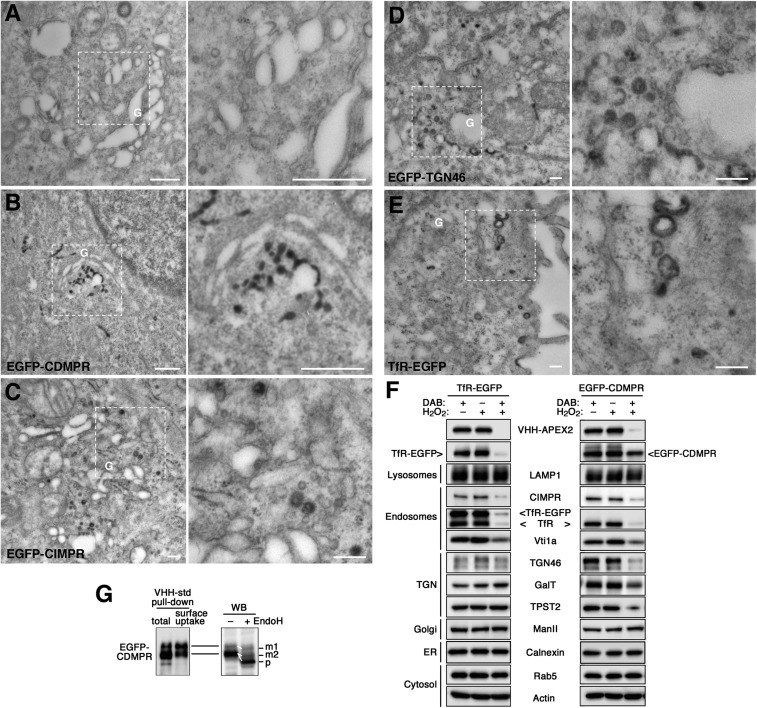
Fig. 4.
Retrograde transport analyzed by APEX2-nanobodies. (A–E) Cytochemical staining of VHH-APEX2–containing compartments for EM. Control and EGFP reporter cell lines were incubated with 5 μg/mL VHH-APEX2 for 1 h (2 h for EGFP-TGN46) at 37 °C to reach steady state. After fixation with 2% glutaraldehyde, cells were treated with DAB/H2O2 to allow DAB polymerization in APEX2-containing compartments, stained with osmium tetroxide and then uranyl acetate, and further processed for EM and sectioned. The panel on the Right is an enlargement of the selected area in the image on the left. (Scale bars, 500 nm.) G, Golgi. (F) Biochemical ablation of VHH-APEX2–containing compartments. To inactivate compartments reached by the TfR-EGFP or EGFP-CDMPR from the cell surface, cells were loaded with VHH-APEX2 for 1 h at 37 °C and washed before incubation with 1 mg/mL DAB, 0.03% H2O2, or both for 90 min at 4 °C. Cells were lysed and subjected to immunoblot analysis to detect the nanobody, the EGFP-target proteins as well as compartment-specific endogenous markers. ManII, α-mannosidase II. Experiments have been repeated three times with consistent results. (G, Left) VHH-std was added either to EGFP-CDMPR cell lysate to bind total receptor protein (total) or to intact cells for 1 h at 37 °C to capture only protein cycling to the surface (surface uptake). VHH-std was then isolated with Ni-NTA beads before immunoblotting for GFP. All of the upper band but only a fraction of the lower one was captured by internalized nanobodies. (Right) Lysate of EGFP-CDMPR expressing cells was treated with or without endoglycosidase H (EndoH) before Western blotting (WB). The upper band and part of the lower one were partially EndoH-resistant (reduced in apparent size by ∼3 kDa), as expected for the mature proteins (m1 and m2). The rest of the lower band showed a larger shift (∼10 kDa), indicating complete Endo-H sensitivity of the precursor (p) still in the ER.