Figure 4.
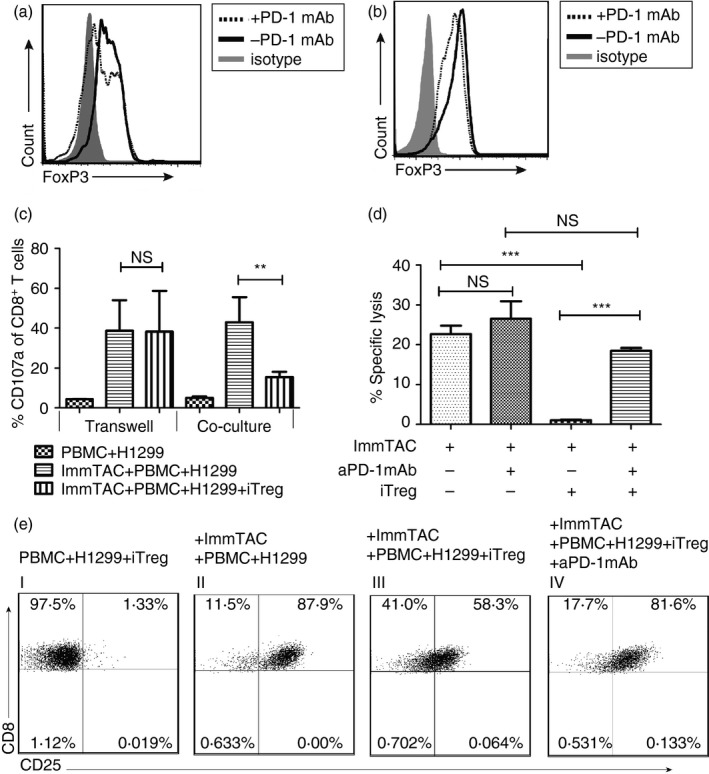
Anti‐programmed cell death protein 1 (PD‐1) monoclonal antibody (mAb) could reverse the suppressive effect of induced regulatory T (iTreg) cells on ImmTAC‐NYE‐redirected cell killing. (a) The Foxhead box protein 3 (FoxP3) expression of the iTreg cells was down‐regulated by an anti‐PD‐1 mAb (10 μg/ml) (black dotted line) during the induction of CD4+ CD45RA + CD25− T cells with the ‘induction cocktail’ (IC), in comparison with the absence of the antibody (black solid line). (b) The anti‐PD1 antibody could destabilize the expression of FoxP3 in the iTreg cells. After a complete induction, the induced iTreg cells was cultured with (black dotted line) or without (black solid line) anti‐PD‐1 mAb (10 μg/ml) for a further 2 days, then the FoxP3 expression was detected by FACS. The grey dashed area was an isotype control for the FoxP3 antibody. (c) Comparison of the iTreg cell inhibition of the CD107a expression by the ImmTAC‐NYE‐stimulated T cells in the transwell and co‐culture system. The cytotoxic T‐lymphocytes (CTL) assays; CTLs in the presence of iTreg cells [ratio of peripheral blood mononuclear cell (PBMC) to iTreg cells = 1 : 1] were cultured in 96‐well transwell or round‐bottom plates (co‐culture), 48 hr later, cells were collected to detect CD107a expression after gating the CD8‐positive cells by FACS. (d) The anti‐PD‐1 antibody reversed the iTreg cell suppressive effects on the ImmTAC‐NYE redirected tumour cell killing. After the anti‐PD‐1 antibody (10 μg/ml) was added to the CTL assays in the presence of iTreg cells (PBMC : iTreg cells = 1 : 1) over a 24‐hr period, lactate dehydrogenase (LDH) release was detected. Unpaired two‐tailed t‐test, ***P < 0·001, NS P > 0·05. (e) The anti‐PD‐1 mAb enhanced CD25 expression of CD8+ T cells suppressed by the iTreg cells. The cultured cells in the CTL assays were collected for the detection of the CD25 expression by CD8+ T cells after gating on CD8+ T cells by staining with anti‐CD8 antibody.
