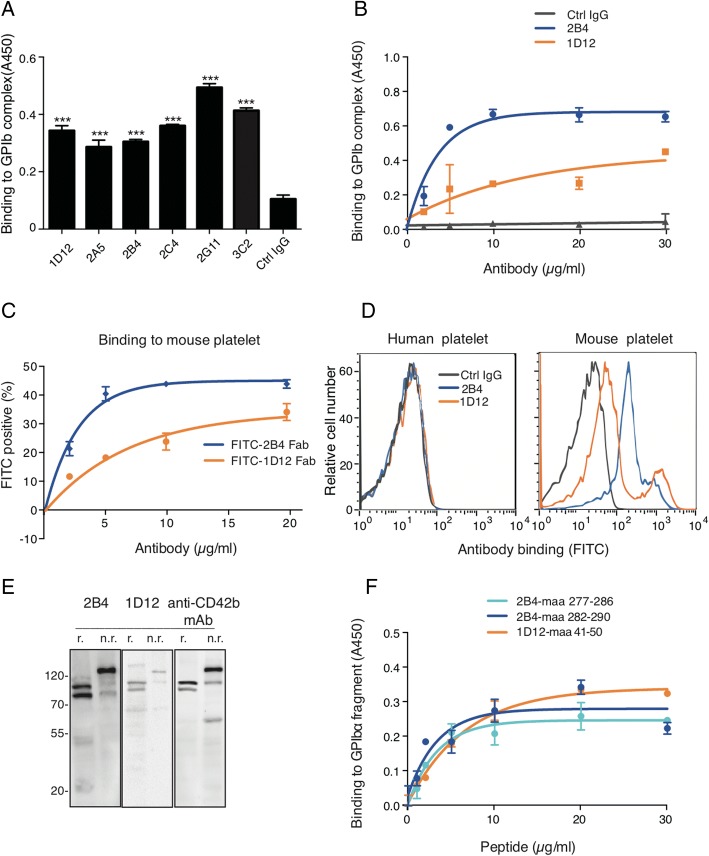
Fig. 1.
2B4 and 1D12 specifically bind to mouse glycoprotein (GP)Ibα. a Binding of rat anti-mouse antibodies to GPIb-IX complex was detected in ELISA. GPIb-IX was captured by anti-GPIX antibody which complex was immobilized in microtiter plates. Supernatant of hybridoma cells, each identified by the clone name, and the negative control, in the form of RPMI-1640 fetal bovine culture medium with 5 μg/ml rat IgG, were added to the coated wells. The bound Ab was detected with HRP-conjugated rabbit anti-rat IgG. ***P < 0.001. b Binding of 2B4 and 1D12 to GPIb-IX complex were detected in ELISA. Purified mAbs, colored as indicated, and negative controls, in the form of rat IgG, were added to the GPIb-IX immobilized microtiter plates. The bound Ab was detected with HRP-conjugated rabbit anti-rat IgG. c Binding of FITC-conjugated Fab to washed mouse platelets was detected in flow cytometry. Washed mouse platelets were incubated with each Fab at indicated concentration. Binding of Fab was detected by flow cytometry and quantitated by mean fluorescence intensity. d 2B4 and 1D12 specifically bound to mouse platelets. Washed human or mouse platelets were incubated with 10 μg/ml FITC-conjugated 2B4 or 1D12, respectively. Binding of Ab was detected by flow cytometry and quantitated by mean fluorescence intensity. e 2B4 and 1D12 recognized specifically GPIbα in Western blot under nonreducing (n.r.) and reducing (r.) conditions. Total lysates of mouse platelets were immunoblotted with either 2B4 or 1D12. Molecular weight marker (M) was shown and labeled in kDa on the left. f Binding of 5 μg/ml of 2B4 or 1D12 to indicated concentration of GPIbα peptide fragments were detected in ELISA. GPIbα peptide fragment was immobilized in microtiter plates. Indicated concentration antibodies were added to the coated wells. The bound Ab was detected with HRP-conjugated rabbit anti-rat IgG. Each figure or histogram is a representative of three independent experiments