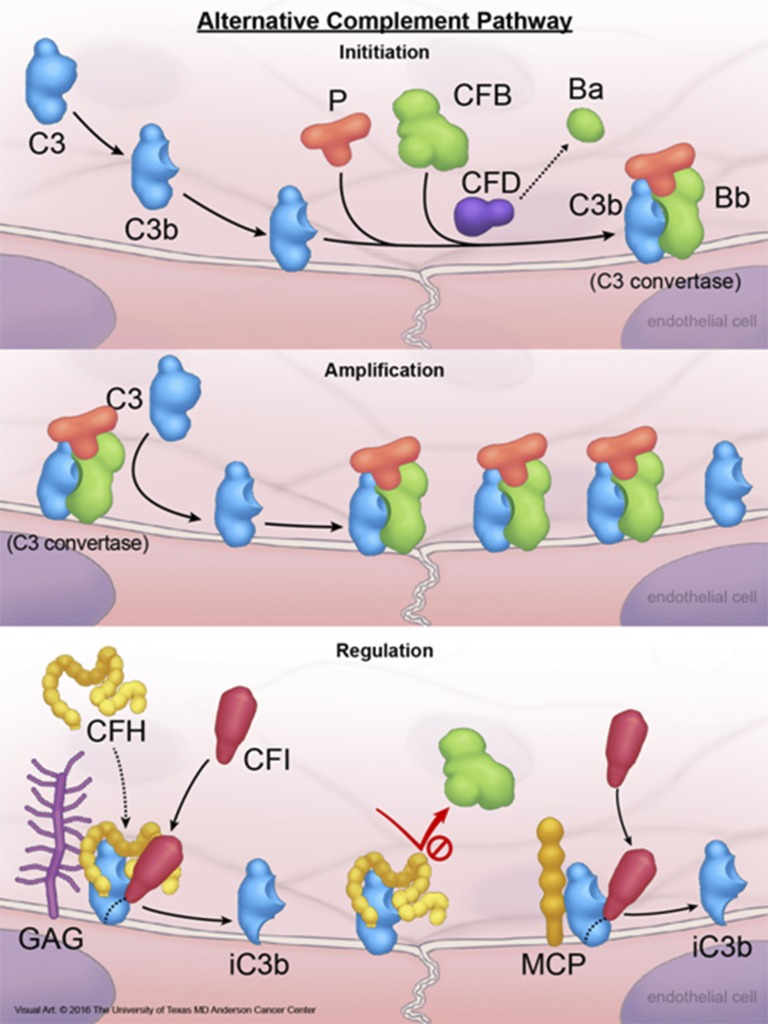
Figure 1.
Activation and regulation of the alternative complement pathway. A small amount of plasma C3 is continuously converted to C3b that is highly active and binds to cell surface via forming thiol bonds. Under normal conditions, C3b is rapidly degraded in plasma by FI and FH (FI cofactor), or on the cell surface by FI and glycosoaminoglycan-bound FH, membrane protein CD46 (or MCP), or CD35. Degradation of C3b to an inactive product (iC3b) on the cell membrane by membrane-bound FH, MCP, or CD35 requires FI, and is known as cofactor activity. IC3b cannot participate in any further complement activation. If not inactivated, C3b is able to bind to an activation product of FB (Bb) produced by FD-mediated cleavage of FB, and generate C3bBb (C3 convertase). C3 convertase is the engine of the complement pathways and deposit additional C3b molecules on membrane, which in turn amplify complement activation. C3 convertase is an important target for complement regulatory proteins. FH prevents the formation of C3 convertase and dissociate C3 convertase by competing with FBb for binding to C3b. This negative regulatory activity of FH is known as decay accelerating activity. If C3bBb remains intact, the complex is stabilized by FP (properdin) and binds to additional C3b to generate C3bBbC3b, which is also known as C5 convertase. C5 convertase activates C5 to generate C5b, which in turn binds to C6, C7, and C8, forming the C5b-8 complex that stably inserts into the lipid bilayer of the cell. Next, the C5b-8 complex binds and polymerizes multiple molecules of C9 (C5b-9n), forming cytolytic MAC of complement on the cell surface. CFB, factor B; CFD, factor D; CFH, factor H; CFI, factor I; GAG, glycosoaminoglycans; MAC, membrane attack complex; P, properdin. Reprinted from The University of Texas MD Anderson Cancer Center with permission.