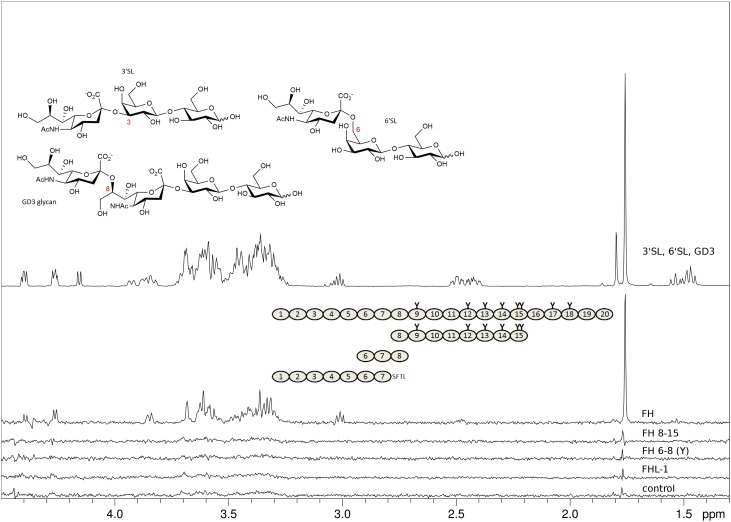
Fig. 2.
FHL-1 and recombinant fragments of FH devoid of CCPs 19–20 do not bind to sialylated glycans. Representatives of α2-3, α2-6, and α2-8-linked Neu5Ac (3’SL, 6’SL, and the GD3 glycan, respectively, chemical structures shown with Neu5Ac glycosidic linkage types in red) were measured at 2 mM each and STD NMR experiments were recorded at 283 K in PBS containing D2O instead of H2O with different proteins at around 10 μM present. Spectra from top to bottom: proton 1D reference spectrum of the sugar mix (top), STD NMR difference spectra with FH (second from top), with FH8-15 (third from top), with FH6–8 402Y (fourth from top), with FHL-1 (fifth from top), and, for comparison, without protein (bottom). Proteins used are shown schematically with naturally occurring glycosylation sites highlighted as “Y”. All recombinantly produced proteins (i.e., all apart from FH) were deglycosylated with Endo Hf during the purification procedure. The FHL-1 C-terminus (deviation from FH sequence) is spelled out as amino acid sequence. The STD NMR difference spectrum with FH is the only STD spectrum with noticeable magnetization transfer to one of the three sialoglycans (signals belong to 3’SL proton resonances).