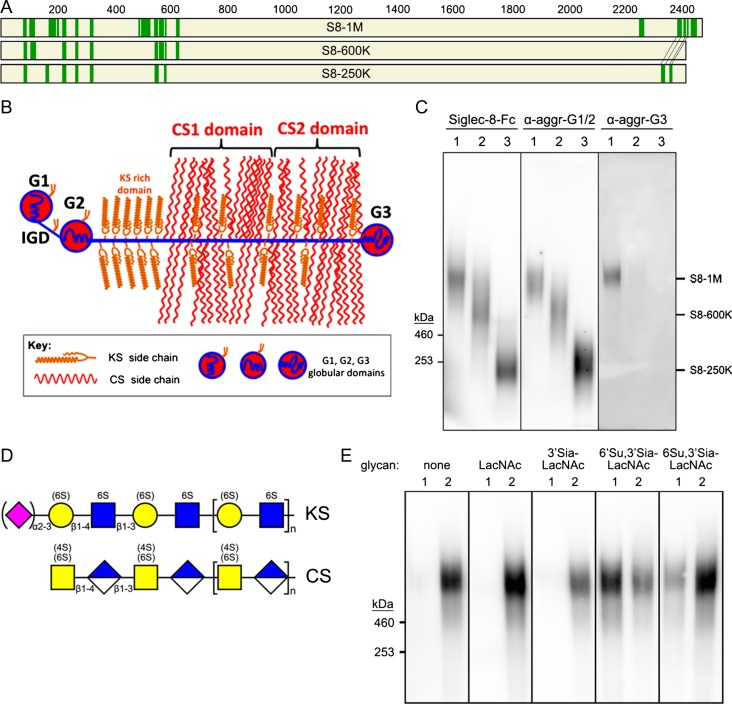
Fig. 4.
Aggrecan carries Siglec-8-binding glycans. (A) Proteins extracted from human trachea were purified by sequential size-exclusion and Siglec-8 affinity chromatography, proteolyzed, and peptides identified by mass spectrometry. Maps of aggrecan protein sequences with identified peptides from three size classes of affinity-purified Siglec-8 ligands are shown. Green bars are peptides (see Table I) that exceed strict false discovery rates for each of the Siglec-8 ligand size classes as indicated. The smaller size classes (S8-600 K and S8-250K) are mapped on aggrecan Uniprot reference sequence P16112 (2415 amino acids); whereas the largest size class (S8-1M) is mapped on C-terminal alternatively spliced aggrecan sequence H0YM81 (2492 amino acids). (B) Schematic map of aggrecan. IGD, interglobular domain; CS1, CS2, chondroitin sulfate (CS)-rich domains. Modified from Caterson and Melrose (2018), with permission. (C) Co-migration of purified human Siglec-8 ligands and aggrecan immunoreactivity. Three purified size classes of Siglec-8 ligands from human trachea were resolved by electrophoresis on replicate 1.5% acrylamide, 2% agarose composite gels, blotted to PVDF membranes and probed with Siglec-8-Fc, anti-aggrecan antibody 7D4 (α-aggr-G1/2) or anti-aggrecan antibody PA1-1745 (α-aggr-G3). Migration positions of HiMark-prestained standards are indicated at the left. Lanes: (1) S81M; (2) S8-600K and (3) S8-250K. (D) Generalized schematic structures of KS and CS chains depicted using symbol nomenclature (Varki et al. 2015). Sialic acid and sulfates that are variable are shown in parentheses. (E) Elution of S8-1M from Siglec-8-COMP affinity chromatography with soluble glycans. Pooled size-exclusion fractions containing S8-1M (Figure 2) were captured on Siglec-8-COMP magnetic beads. The beads were thoroughly washed prior to eluting with β-azidoethylglycosides (lane 1) followed by 500 mM imidazole to elute the bound Siglec-8-COMP with any remaining ligand attached (lane 2). Eluates were resolved by composite gel electrophoresis, blotted and probed with Siglec-8-Fc. Elution was tested with the following β-azidoethylglycosides: none, N-acetyllactosamine (LacNAc, Galβ1-4GlcNAc), 3′-sialyl LacNac (3′Sia-LacNAc, Neu5Acα2-3Galβ1-4GlcNAc), 6′-sulfo-3′-sialyl-LacNAc (6′Su,3′Sia-LacNAc, Neu5Acα2-3[6 S]Galβ1-4GlcNAc) and 6-sulfo-3′-sialyl-LacNAc (6 Su,3′Sia-LacNAc, Neu5Acα2-3Galβ1-4[6S]GlcNAc).