This population-based cohort study examines the association between gestational age at birth and symptoms of attention-deficit/hyperactivity disorder among children at 5 and 8 years of age and possible sex differences in the associations.
Key Points
Questions
Is the association between gestational age at birth and symptoms of attention-deficit/hyperactivity disorder the same at 5 and 8 years of age, and are there possible sex differences in the associations?
Findings
In this population-based cohort study of 113 227 children that used a sibling comparison approach to adjust for confounding, an association was found between early preterm birth (gestational age <34 weeks) and symptoms of attention-deficit/hyperactivity disorder in preschool and school-age children.
Meaning
The findings illustrate potential gains of reducing preterm birth and the importance of providing custom support to children born preterm to prevent neurodevelopmental problems.
Abstract
Importance
Preterm birth is associated with an increased risk of attention-deficit/hyperactivity disorder (ADHD); however, it is unclear to what extent this association can be explained by shared genetic and environmental risk factors and whether gestational age at birth is similarly related to inattention and hyperactivity/impulsivity and to the same extent in boys and girls.
Objectives
To investigate the association between gestational age at birth and symptoms of ADHD in preschool and school-age children after adjusting for unmeasured genetic and environmental risk factors.
Design, Setting, and Participants
In this prospective, population-based cohort study, pregnant women were recruited from across Norway from January 1, 1999, through December 31, 2008. Results of a conventional cohort design were compared with results from a sibling-comparison design (adjusting for genetic and environmental factors shared within families) using data from the Norwegian Mother and Child Cohort Study. Data analysis was performed from October 1, 2017, through March 16, 2018.
Exposures
Analyses compared children and siblings discordant for gestational age group: early preterm (delivery at gestational weeks 22-33), late preterm (delivery at gestational weeks 34-36), early term (delivery at gestational weeks 37-38), delivery at gestational week 39, reference group (delivery at gestational week 40), delivery at gestational week 41, and late term (delivery after gestational week 41).
Main Outcomes and Measures
Maternally reported symptoms of ADHD in children at 5 years of age and symptoms of inattention and hyperactivity/impulsivity at 8 years of age. Covariates included child and pregnancy characteristics associated with the week of delivery and the outcomes.
Results
A total of 113 227 children (55 187 [48.7%] female; 31 708 [28.0%] born at gestational week 40), including 33 081 siblings (16 014 female [48.4%]; 9705 [29.3%] born at gestational week 40), were included in the study. Children born early preterm were rated with more symptoms of ADHD, inattention, and hyperactivity/impulsivity than term-born children. After adjusting for unmeasured genetic and environmental factors, children born early preterm had a mean score that was 0.24 SD (95% CI, 0.14-0.34) higher on ADHD symptom tests, 0.33 SD (95% CI, 0.24-0.42) higher on inattention tests, and 0.23 SD (95% CI, 0.14-0.32) higher on hyperactivity/impulsivity tests compared with children born at gestational week 40. Sex moderated the association of gestational age with preschool ADHD symptoms, and the association appeared to be strongest among girls. Early preterm girls scored a mean of 0.8 SD (95% CI, 0.12-1.46; P = .02) higher compared with their term-born sisters.
Conclusions and Relevance
After accounting for unmeasured genetic and environmental factors, early preterm birth was associated with a higher level of ADHD symptoms in preschool children. Early premature birth was associated with inattentive but not hyperactive symptoms in 8-year-old children. This study demonstrates the importance of differentiating between inattention and hyperactivity/impulsivity and stratifying on sex in the study of childhood ADHD.
Introduction
Low gestational age at birth is associated with an increased risk of attention-deficit/ hyperactivity disorder (ADHD) and symptoms of ADHD in childhood,1,2,3 as recently summarized in a meta-analysis.4 Previous studies have mainly focused on the consequences of being born extremely (before gestational week 26)4,5,6 or very (before gestational week 32)4,7,8,9,10 preterm. However, increased risk has also been found for children born moderately preterm,3,11 and a previous study12 suggests that each additional week inside the womb is associated with a decrease in risk of ADHD.
Although the association between prematurity and ADHD is well established, it is uncertain to what extent this association is attributable to confounding factors, that is, third variables that influence both the dependent and the independent variable, causing a spurious association. Conventional association analyses include measured covariates to rule out confounding. However, confounding could be attributable to unmeasured factors, for example, genetic or shared environmental factors. Most prior studies4,12 on gestational age and ADHD have not been able to rule out such confounding.
A sibling-comparison approach takes advantage of the fact that full siblings share stable aspects of the familial context, including the same mother during pregnancy, as well as half their genome. In sibling analyses, these unmeasured factors are adjusted for, as are the measured covariates that vary across pregnancies. Previous sibling studies have investigated the association between gestational age and ADHD diagnoses11 and medication use.3 On the basis of more than 1 million siblings from a Swedish cohort, their results indicated that preterm and early term birth increases the risk of ADHD.3,11
Studies13,14,15 based on various levels of measurement (eg, biological, phenotypic, and genetic) suggest a partly different origin for the 2 core ADHD symptom dimensions of inattention and hyperactivity/impulsivity. To our knowledge, a sibling-comparison approach has not yet been applied to investigate gestational age in relation to these dimensions separately. Some conventional association studies5,8,16,17,18,19 indicate that gestational age influences the inattentive more than the hyperactive symptoms; however, this finding was not suggested by a recent meta-analysis.4 Treating ADHD as continuously distributed dimensions increases the power of a sibling design. Use of symptom scales as outcomes also enables comparison across age groups. Because ADHD is usually diagnosed after the age of 6 years, the associations among preschool children are better investigated with dimensional measures.
The prevalence of ADHD is higher in boys than in girls, and the disorder manifests differently because a larger proportion of girls display inattentive symptoms.20,21 Because of these differences, it is important to investigate whether sex moderates the association between gestational week at birth and ADHD symptoms. The aims of this study were to examine the association between gestational age at birth and symptoms of ADHD at 5 years of age, investigate whether gestational age is similarly associated with inattention and hyperactivity/impulsivity at 8 years of age, investigate to what extent the associations can be explained by unmeasured genetic and environmental factors, and examine possible sex differences in the associations.
Methods
The Norwegian Mother and Child Cohort Study (MoBa) is a prospective, population-based cohort study conducted by the Norwegian Institute of Public Health, with data on more than 113 000 mother-child dyads. Pregnant women were recruited from across Norway from January 1, 1999, through December 31, 2008, after attending a routine ultrasonography examination. The participation rate was 41%. Data analysis was performed from October 1, 2017, through March 16, 2018. A detailed description of the sample and data collection is provided elsewhere,22,23 and questionnaires are available online (https://www.fhi.no/en/studies/moba/). Written informed consent was obtained from all participating women. All data were deidentified. MoBa has obtained a license from the Norwegian Data Inspectorate and approval from the Regional Committee for Medical Research Ethics. This study was approved by the Regional Committee for Medical Research Ethics and is based on version 10 of the quality-assured data files released for research in 2017.
Questionnaire data collected at the 17th and 30th weeks of gestation and 6 months after birth provide information on pregnancy-specific variables. When the children were 5 and 8 years of age, questionnaires, including the outcome scales for this study, were mailed to the mothers. The MoBa data have been linked to data from the Medical Birth Registry of Norway, originating from mandatory notification forms completed by midwives, obstetricians, and pediatricians. Among several medical variables, the gestational age at birth is registered.
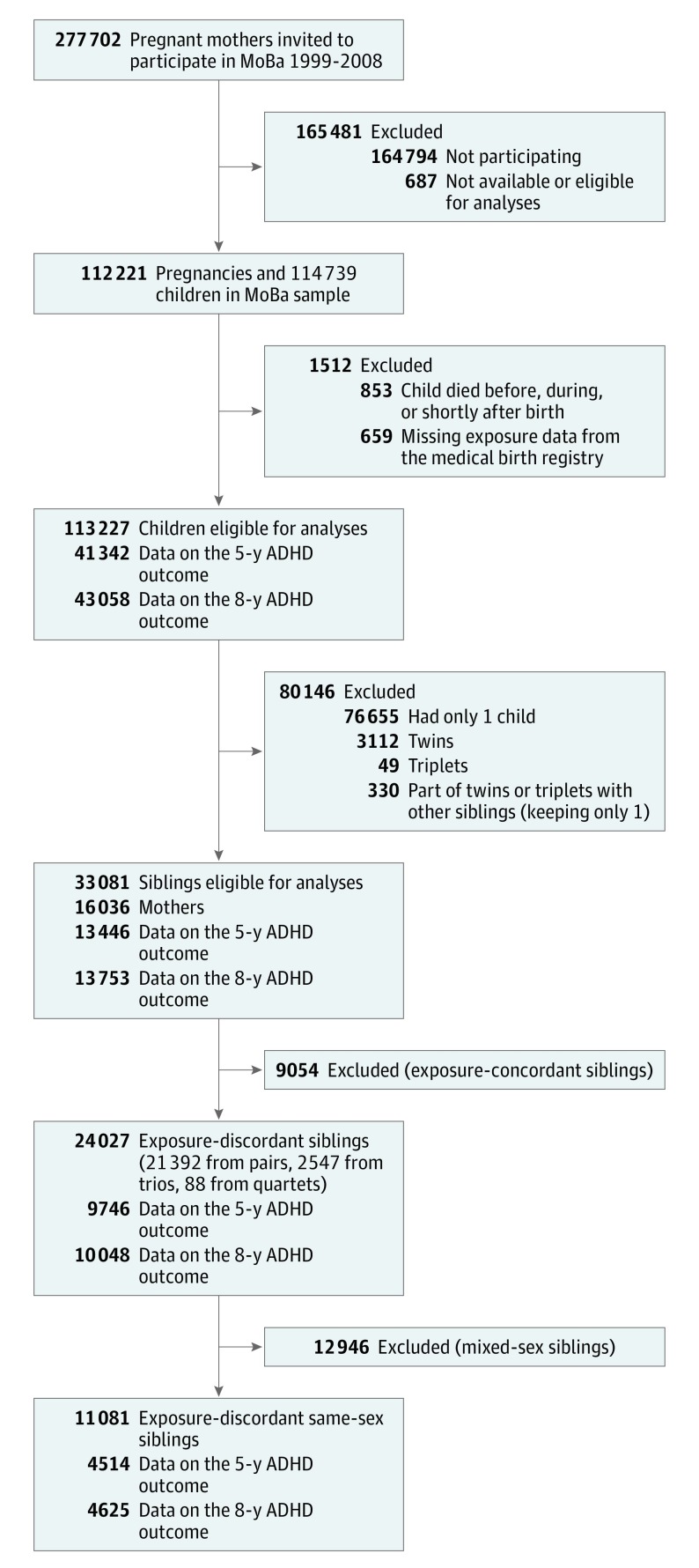
Approximately 18 000 mothers participate in MoBa with more than 1 child, resulting in data on siblings. The flowchart in Figure 1 describes the selection of participants for the various steps of our analyses. Descriptive characteristics for the samples are included in eTable 1 in the Supplement.
Figure 1. Flow Diagram.
Participants included in conventional cohort analyses (steps 1 and 2), sibling comparison analyses (step 3), and sex-stratified sibling comparison (step 4). ADHD indicates attention-deficit/hyperactivity disorder; MoBa, Norwegian Mother and Child Cohort Study.
Outcome Definitions
In the 5-year-old children, symptoms of ADHD were assessed using 12 items from the Conner’s Parent Rating Scale–Revised.24 The items reflect criteria for ADHD in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV)25 (eg, short attention span). Mothers reported how much each symptom had been a problem for the child during the past month using a 4-point scale (with 0 indicating not true and 3 indicating very much true). Factor analysis indicated an acceptable fit of a 1-factor solution (root mean square error of approximation [RMSEA] = 0.068, comparative fit index [CFI] = 0.96).
In the 8-year questionnaire, symptoms were measured using ADHD-related DSM-IV items from the Parent/Teacher Rating Scale for Disruptive Behavior Disorders26: 9 symptoms of inattention and 9 symptoms of hyperactivity/impulsivity. Each item was rated on a 4-point scale (with 1 indicating never/rarely and 4 indicating very often). Exploratory and confirmatory factor analysis supported a 2-factor solution (RMSEA = 0.068, CFI = 0.96) as opposed to 1 underlying ADHD factor (RMSEA = 0.113, CFI = 0.086).
Three latent factors reflecting symptoms of ADHD at 5 years of age, inattention at 8 years of age, and hyperactivity/impulsivity at 8 years of age were therefore used as outcomes in the analyses. The skewness of the single items was high (range, 0.63-3.39), with mothers typically rating children on the lowest categories. Therefore, the items were treated as categorical indicators of the latent variables to which normal distribution is assumed. The use of latent outcome variables maximizes the covariance between the questionnaire items and minimizes the variance caused by measurement error.27 Multigroup confirmatory factor analyses indicated that the factor loadings and variances were similar for boys and girls.
Exposures
Gestational age at birth was based on ultrasonography findings, and the children were categorized according to gestational age. The ends of the distribution were combined into early preterm (delivery at gestational weeks 22-33), late preterm (delivery at gestational weeks 34-36), early term (delivery at gestational weeks 37-38), and late term (delivery at gestational week >41), according to previous classifications.18,28
Covariates
On the basis of the previous literature,28,29,30 the confounding potential of several variables was explored. Variables with a significant association with gestational age and 1 of the outcomes were included in the adjusted models: sex, multiple birth status, being small for gestational age (2-SD difference from the uterine growth curve31), serious congenital malformations, parity, and bleeding before gestational week 13. A detailed description of the covariate selection is provided in the eMethods, eTable 2, and eTable 3 in the Supplement.
Statistical Analysis
Analyses were performed in Mplus, version 8.32 Because list-wise deletion of cases with incomplete data can increase sample bias,33 the full information maximum likelihood estimator was used for handling missing outcome data under a missing at random expectation. This approach is recommended because it makes use of all available data.33 On the basis of structural equation modeling, P < .05 (2-sided) was considered to be statistically significant.
Unadjusted and adjusted regression models for each outcome were run in 3 steps, all including gestational age as a categorical exposure variable (gestational week 40 as reference group). The first set of analyses examined the overall associations between gestational age and ADHD symptoms in the total sample (step 1). A second set of analyses estimated the same overall associations in the sibling sample (step 2). In steps 1 and 2, structural equation models with 1 latent outcome were used to estimate the regression paths of gestational age and measured covariates (eFigure in the Supplement). Nonindependence between sibling observations was accounted for in the study.
The third set of analyses involved comparing exposure-discordant siblings (step 3). The main goal was to address to what extent the associations observed in conventional analyses can be explained by unmeasured familial factors (ie, the green area in eFigure in the Supplement). Factors shared by siblings comprise all stable risk factors in their mother (eg, 100% of genetic risk for preterm delivery) and 50% of genetic risk for ADHD deriving from the fetus. By extracting the variance explained by unmeasured shared factors, we get closer to identifying the origin of the association. Technically, the variance attributable to shared factors was extracted by including a second level in the models, in which the variance explained by family mean levels of ADHD symptoms was estimated. This analytic approach is described in detail elsewhere.28
Exposure-outcome regression coefficients were estimated for each gestational group, indicating how much each group differed from the reference group. The coefficients were standardized by dividing them by the square root of the total variance in the latent outcome. Standardized coefficients represent mean SD differences and are equivalent to the Cohen d effect size.
The interpretation of SD differences can be illustrated by picturing 2 normal distributions on an x-axis, 1 for the control group and 1 for the early premature group. An SD difference of, for example, 0.3 indicates that the premature distribution is moved 0.3 SD along the x-axis. The standardized mean value for this group is no longer 0 but 0.3. It is possible to calculate the percentage of the exposure distribution that is above the mean of the control group. In our example (0.3 SD), this amount would be 62%. Using an assumed cutoff for a dichotomous outcome (eg, that 5% of children in the population have ADHD), we can calculate how many more children would have ADHD in the exposure vs the control group. In our example, there would be 3.9% more children with ADHD in the early premature group vs the control group. To illustrate the size of the SD difference estimates, these numbers can be converted to odds ratios (ORs) using the formula Log OR = Cohen d (π/3).34
If estimated differences identified in steps 1 and 2 were also present in the sibling-comparison analysis, it is more likely that young gestational age increases the risk of ADHD symptoms. If the differences were attenuated or disappeared in the sibling-control, the most plausible explanation is that the association is partly or fully explained by unmeasured confounders.
To investigate sex differences, sex × gestational age interaction terms were tested in the total sample. Gestational age was included both as a continuous and squared indicator (to account for nonlinearity), and 2 corresponding interaction terms were tested. Sex-stratified analyses were performed for outcomes with a significant interaction term (ADHD-5).
Results
A total of 113 227 children (55 187 [48.7%] female; 31 708 [28.0%] born at gestational week 40), including 33 081 siblings (16 014 female [48.4%]; 9705 [29.3%] born at gestational week 40), were included in the study. Mean values on the raw outcome scores by gestational age are presented in eTable 4 in the Supplement (stratified by sex in eTable 5 in the Supplement). Results of the conventional analyses of the total sample are presented in Table 1 (5 years) and Table 2 (8 years). Children born early preterm had a mean score that was 0.24 SD (95% CI, 0.14-0.34) higher on ADHD at 5 years of age, 0.33 SD (95% CI, 0.24-0.42) higher on inattention at 8 years of age, and 0.23 SD (95% CI, 0.14-0.32) higher on hyperactivity at 8 years of age compared with children born at gestational week 40. Corresponding ORs were 1.55 (95% CI, 1.29-1.85) for ADHD at 5 years of age, 1.85 (95% CI, 1.55-2.14) for inattention at 8 years of age, and 1.52 (95% CI, 1.29-1.79) for hyperactivity at 8 years of age. Adjusted estimates were 0.15 SD (95% CI, 0.05-0.25) for ADHD at 5 years of age, 0.31 SD (95% CI, 0.21-0.41) for inattention at 8 years of age, and 0.16 SD (95% CI, 0.07-0.25) for hyperactivity at 8 years of age.
Table 1. Standardized Differences in ADHD Symptoms Among 5-Year-Old Children by Gestational Age Group.
| Gestational Week | SD (95% CI) | |||||
|---|---|---|---|---|---|---|
| Total MoBa Samplea | Total Sibling Sampleb | Sibling Comparison Modelc | ||||
| Unadjusted | Adjusted | Unadjusted | Adjustedd | Unadjusted | Adjustedd | |
| <34 | 0.24 (0.14 to 34.0) | 0.15 (0.05 to 25.0) | 0.28 (0.07 to 49.0) | 0.27 (0.06 to 48.0) | 0.42 (0.12 to 72.0) | 0.32 (0.02 to 62.0) |
| 34-36 | 0.07 (0.01 to 13.0) | 0.04 (−0.02 to 10.0) | 0.09 (−0.03 to 21.0) | 0.10 (−0.02 to 22.0) | 0.09 (−0.06 to 24.0) | 0.02 (−0.14 to 18.0) |
| 37-38 | −0.01 (−0.05 to 3.0) | 0.02 (−0.02 to 6.0) | 0 (−0.06 to 6.0) | 0.04 (−0.02 to 10.0) | 0.03 (−0.05 to 11.0) | 0.03 (−0.05 to 11.0) |
| 39 | −0.04 (−0.07 to −0.01) | −0.01 (−0.04 to 2.0) | 0.03 (−0.02 to 8.0) | 0.06 (0.01 to 11.0) | 0.04 (−0.03 to 11.0) | 0.06 (−0.01 to 13.0) |
| 40 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 41 | −0.02 (−0.06 to 2.0) | −0.02 (−0.05 to 1.0) | 0.01 (−0.04 to 6.0) | 0.01 (−0.04 to 6.0) | 0 (−0.07 to 7.0) | −0.05 (−12.0 to 2.0) |
| >41 | 0.04 (−0.01 to 9.0) | −0.02 (−0.07 to 3.0) | 0.16 (0.08 to 24.0) | 0.10 (0.02 to 18.0) | 0.10 (0 to 20.0) | −0.03 (0.13 to 7.0) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; MoBa, Norwegian Mother and Child Cohort Study.
N = 113 227 (41 342 with outcome data).
n = 33 081 (13 446 With outcome data).
n = 24 027 Siblings discordant on exposure (9315 with outcome data [discordant]). The full information maximum likelihood estimator was used for handling missing data.
Adjusted for pregnancy-specific characteristics: sex, small for gestational age, congenital malfunctions, parity, plurality, and bleeding before gestational week 13.
Table 2. Standardized Differences in Inattention and Hyperactivity Among in 8-Year-Old Children by Gestational Age Groups.
| Gestational Week | SD (95% CI) | |||||
|---|---|---|---|---|---|---|
| Total MoBa Samplea | Total Sibling Sampleb | Sibling Comparison Modelc | ||||
| Unadjusted | Adjusted | Unadjusted | Adjustedd | Unadjusted | Adjustedd | |
| Inattention | ||||||
| <34 | 0.33 (0.24 to 42.0) | 0.31 (0.21 to 41,0) | 0.34 (0.13 to 0.55) | 0.32 (0.12 to 52.0) | 0.31 (0.05 to 57.0) | 0.31 (0.05 to 57.0) |
| 34-36 | 0.09 (0.03 to 15.0) | 0.11 (0.05 to 17.0) | 0.08 (−0.03 to 19.0) | 0.07 (−0.03 to 17.0) | 0.03 (−0.11 to 17.0) | −0.02 (−0.16 to 12.0) |
| 37-38 | 0.01 (−0.02 to 4.0) | 0.03 (0 to 6.0) | 0.02 (−0.05 to 9.0) | 0.05 (−0.01 to 11.0) | 0.01 (−0.07 to 9.0) | 0.04 (−0.04 to 12.0) |
| 39 | −0.04 (−0.07 to −0.01) | −0.02 (−0.05 to 1.0) | 0.01 (−0.06 to 8.0) | 0.03 (−0.02 to 8.0) | 0 (−0.06 to 6.0) | 0.03 (−0.03 to 9.0) |
| 40 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 41 | −0.01 (−0.04 to 2.0) | −0.03 (−0.06 to 0) | 0.04 (−0.03 to 11.0) | 0.03 (−0.02 to 8.0) | 0.03 (−0.04 to 10.0) | −0.01 (−0.08 to 6.0) |
| >41 | 0.06 (0.02 to 10.0) | −0.01 (−0.05 to 3.0) | 0.11 (0.03 to 19.0) | 0.06 (−0.01 to 13.0) | 0.12 (0.02 to 22.0) | 0 (−0.10 to 10.0) |
| Hyperactivity | ||||||
| <34 | 0.23 (0.14 to 32.0) | 0.16 (0.07 to 25.0) | 0.29 (0.08 to 0.50) | 0.28 (0.07 to 49.0) | 0 (−0.28 to 28.0) | −0.03 (−0.32 to 26.0) |
| 34-36 | 0.11 (0.05 to 17.0) | 0.08 (0.02 to 14.0) | 0.09 (−0.13 to 0.31) | 0.07 (−0.04 to 18.0) | 0.05 (−0.09 to 19.0) | 0.01 (−0.13 to 15.0) |
| 37-38 | 0.01 (−0.02 to 4.0) | 0.02 (−0.01 to 5.0) | 0.05 (−0.01 to 0.11) | 0.06 (0 to 12.0) | 0.02 (−0.06 to 10.0) | 0.04 (−0.04 to 12.0) |
| 39 | −0.04 (−0.07 to −0.01) | −0.02 (−0.05 to 2.0) | 0.01 (−0.04 to 0.06) | 0.02 (−0.03 to 7.0) | 0 (−0.07 to 7.0) | 0.02 (−0.05 to 9.0) |
| 40 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 41 | −0.02 (−0.05 to 1.0) | −0.03 (−0.06 to 0) | 0.05 (0 to 0.10) | 0.02 (−0.03 to 7.0) | 0.05 (−0.02 to 12.0) | 0.02 (−0.05 to 9.0) |
| >41 | 0.04 (0 to 8.0) | 0 (−0.04 to 4.0) | 0.12 (0.40 to 0.20) | 0.07 (0 to 14.0) | 0.11 (0.01 to 21.0) | 0.03 (−0.07 to 13.0) |
Abbreviation: MoBa, Norwegian Mother and Child Cohort Study.
N = 113 227 (43 058 with outcome data).
n = 33 081 (13 780 With outcome data).
n = 24 027 Siblings discordant on exposure (9833 with inattention outcome data, 9412 with hyperactivity outcome data [discordant]). The full information maximum likelihood estimator was used for handling missing data.
Adjusted for pregnancy-specific characteristics: sex, small for gestational age, congenital malfunctions, parity, plurality, and bleeding before gestational week 13.
Before sibling control but adjusted for pregnancy-specific risk factors, an association was apparent between early preterm birth and ADHD symptoms in the sibling sample. Children born early preterm scored 0.27 SD (95% CI, 0.06-0.48) higher on ADHD at 5 years of age (Table 1), 0.32 SD (95% CI, 0.12-0.52) higher on inattention at 8 years of age, and 0.28 SD (95% CI, 0.07-0.49) higher on hyperactivity at 8 years of age (Table 2). Corresponding ORs were 1.63 (95% CI, 1.11-2.39) for ADHD at 5 years of age, 1.79 (95% CI, 1.24-2.57) for inattention at 8 years of age, and 1.66 (95% CI, 1.14-2.43) for hyperactivity at 8 years of age.
Familial factors explained 43% of the total variance in ADHD at 5 years of age, 35% of hyperactivity at 8 years of age, and 34% of inattention at 8 years of age. To investigate whether this shared variance accounts for the association between prematurity and ADHD, exposure-discordant siblings were compared. Results are presented in Table 1 and Table 2. Compared with their siblings born in gestational week 40 and adjusted for pregnancy-specific factors, children born early preterm scored 0.32 SD (95% CI, 0.02-0.62) higher on ADHD at 5 years of age, 0.31 SD (95% CI, 0.05-0.57) higher on inattention at 8 years of age, and 0.03 (95% CI, −0.32 to 0.26) lower on hyperactivity at 8 years of age. Corresponding ORs were 1.79 (95% CI, 1.04-3.08) on ADHD at 5 years of age, 1.75 (95% CI, 1.09-2.81) on inattention at 8 years of age, and 0.95 (95% CI, 0.21-1.60) on hyperactivity at 8 years of age. Pregnancy-specific covariates accounted for a small portion (0.10 SD) of the association with ADHD-5 and close to none of the 8-year outcomes.
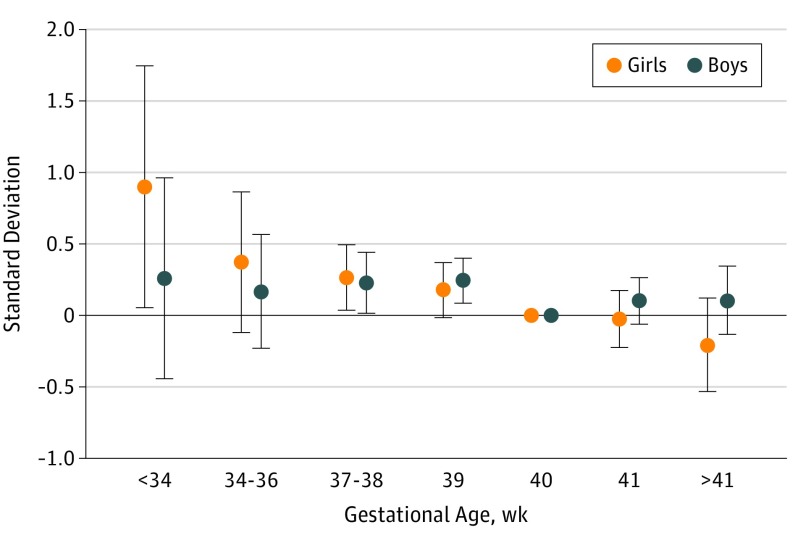
There was a sex by gestational week (linear and quadratic) interaction effect on ADHD symptoms at 5 years of age. Adjusted results of sex-stratified sibling control analyses are presented in Figure 2. Early preterm girls scored a mean of 0.8 SD higher compared with their term-born sisters (95% CI, 0.12-1.46; P = .02), corresponding to an OR of 4.27 (95% CI, 1.24-14.13). Figure 2 indicates a dose-response association between gestational age and ADHD-5 in girls, which was not evident for boys.
Figure 2. Gestational Age at Birth and Attention-Deficit/Hyperactivity Symptoms in 5-Year-Old Boys and Girls.
Dots indicate differences in fractions of SDs compared with same-sex siblings born in gestational week 40. Error bars indicate 95% CIs.
Discussion
The results of the sibling-control approach used in our study suggest that early premature birth increases the risk of symptoms of ADHD in preschool-age children and symptoms of inattention in school-age children. The preschool association was most pronounced among girls. The association between premature birth and hyperactivity/impulsivity was completely confounded by factors shared between siblings. There was no indication of a negative association of being born in gestational weeks 34 to 39 (effect sizes typically <0.1 SD). In addition, the negative association of being born late term was attenuated in the sibling control models.
The observation that confounding attributable to unmeasured factors did not account for the associations with ADHD at 5 years of age and inattention 8 years of age are in line with earlier sibling comparisons based on dichotomous ADHD outcomes3,11 and with another sibling study28 that found neurodevelopmental problems (ie, language delay) in preterm children. In accordance with previous literature,5,8,16,17,18,19 being born preterm is associated with inattention more than with hyperactivity. Such a differential association was not supported by a recent meta-analysis.4 Unfortunately, the preschool outcome measure did not allow differentiating between inattention and hyperactivity. Most of the items of ADHD at 5 years of age tap the dimension of inattention, and the significant results could be driven by an association with this primarily.
Various mechanisms might explain the association between early preterm birth and ADHD and inattention symptoms.1 Several authors3,11 suggest the immaturity of the brain and its development as the main reason. At gestational week 35, the weight of the brain is approximately 60% of that at term,35 and it is in a critical period of development that normally takes place in utero.36 Preterm children are at higher risk for postnatal complications and are often exposed to factors that can promote neuronal cell death in the brain. This could lead to volumetric losses in specific brain regions and may partially explain the cognitive abnormalities in these children.37
Adjusting for measured covariates did not account for a substantial proportion of the association. Although we investigated the confounding potential of a wide range of variables, we cannot rule out the possibility that other pregnancy-specific factors closely associated with gestational age could explain the association between early premature delivery and ADHD symptoms (eg, infection or inflammation).38
Our results suggest that the negative consequences of being born preterm are most pronounced in girls (at 5 years of age), although the power of the sex-stratified analyses is limited. A high score on inattention might be a reflection of related constructs, for example, anxiety, which is more prevalent among girls than boys, a possible explanation for the observed sex difference.
Limitations
There are 5 important limitations of the current study. First, the participation rate was 41%, suggesting the possibility of bias attributable to nonrandom participation. Young women, smokers, and women with low educational level were underrepresented.39 However, bivariate associations do not seem to be affected by the low participation rate in MoBa.39
Second, attrition over time might cause a selection bias if mothers lost to follow-up have a higher rate of preterm deliveries and children with ADHD symptoms. The sibling design represents a robust approach to selection bias because stable selection factors are completely adjusted for. The sibling sample was comparable to the total MoBa sample on most variables, confirming a representative sample.
Third, in the sibling comparisons, only exposure-discordant siblings contribute to the estimated association. This implies a selection of pairs that also differ in possible reasons for being born preterm vs term, including possible confounders. The confounder-exposure association could be strengthened, thus increasing any spurious association attributable to nonshared confounding bias. If siblings are less similar with regard to confounders than to the exposure under study, the sibling-control estimates will be biased.40 However, we believe that gestational age is randomly distributed. The intraclass correlation for gestational age was 0.34 (95% CI, 0.31-0.36). In addition, the measured confounders adjusted for in our analyses do not explain much of the association. It is not likely that the observed associations would be completely attributable to confounders not shared by the siblings.
Fourth, adjustment for unmeasured factors shared among siblings may include adjustment for variables that lie on the pathway from the exposure to the outcome (mediators), possibly introducing bias.41 For example, having a premature infant could influence the family environment (eg, parental distress), in turn influencing the symptom level of all the siblings. However, because the association is not strongly reduced after sibling control, the inclusion of important mediators is not likely.
Fifth, although the items used closely mirror the DSM-IV criteria, maternal reports are not equivalent to a psychiatric evaluation. However, previous research has suggested that ADHD as a disorder is not etiologically different from ADHD as a continuum.42
Conclusions
To our knowledge, this is the first study to investigate the association between preterm birth and symptoms of ADHD using a sibling-comparison design. We found that early premature birth was associated with ADHD symptoms in preschool-age children and inattention symptoms in school-age children. Our study emphasizes the benefit of a sibling-comparison design and shows that differentiating between dimensions of inattention and hyperactivity/impulsivity, as well as by sex, can provide important knowledge about ADHD. The findings illustrate potential gains of reducing preterm birth and the importance of providing custom support to children born preterm to prevent neurodevelopmental problems.
eTable 1. Selected Descriptive Characteristics of the MoBa and Sibling Samples
eTable 2. Potential Covariates Associated With Symptoms of ADHD
eTable 3. Potential Covariates Associated With Gestational Week at Birth
eTable 4. Mean Scores of the ADHD Symptom Scales by Gestational Age at Birth
eTable 5. Sex Stratified Mean Scores of the ADHD Symptom Scales by Gestational Week (GW) at Birth
eMethods. Covariate Selection
eFigure. Illustration of the Difference Between Conventional Cohort Analyses (Step 1 and 2) and Sibling Comparison Analyses (Step 3)
References
- 1.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728-737. doi: 10.1001/jama.288.6.728 [DOI] [PubMed] [Google Scholar]
- 2.Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. 2011;69(5, pt 2):11R-18R. doi: 10.1203/PDR.0b013e318212faa0 [DOI] [PubMed] [Google Scholar]
- 3.Lindström K, Lindblad F, Hjern A. Preterm birth and attention-deficit/hyperactivity disorder in schoolchildren. Pediatrics. 2011;127(5):858-865. doi: 10.1542/peds.2010-1279 [DOI] [PubMed] [Google Scholar]
- 4.Franz AP, Bolat GU, Bolat H, et al. . Attention-deficit/hyperactivity disorder and very preterm/very low birth weight: a meta-analysis. Pediatrics. 2018;141(1):e20171645. doi: 10.1542/peds.2017-1645 [DOI] [PubMed] [Google Scholar]
- 5.Johnson S, Marlow N. Growing up after extremely preterm birth: lifespan mental health outcomes. Semin Fetal Neonatal Med. 2014;19(2):97-104. doi: 10.1016/j.siny.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 6.Scott MN, Taylor HG, Fristad MA, et al. . Behavior disorders in extremely preterm/extremely low birth weight children in kindergarten. J Dev Behav Pediatr. 2012;33(3):202-213. doi: 10.1097/DBP.0b013e3182475287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717-728. doi: 10.1542/peds.2008-2816 [DOI] [PubMed] [Google Scholar]
- 8.Brogan E, Cragg L, Gilmore C, Marlow N, Simms V, Johnson S. Inattention in very preterm children: implications for screening and detection. Arch Dis Child. 2014;99(9):834-839. doi: 10.1136/archdischild-2013-305532 [DOI] [PubMed] [Google Scholar]
- 9.Delobel-Ayoub M, Arnaud C, White-Koning M, et al. ; EPIPAGE Study Group . Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123(6):1485-1492. doi: 10.1542/peds.2008-1216 [DOI] [PubMed] [Google Scholar]
- 10.Treyvaud K, Ure A, Doyle LW, et al. . Psychiatric outcomes at age seven for very preterm children: rates and predictors. J Child Psychol Psychiatry. 2013;54(7):772-779. doi: 10.1111/jcpp.12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Onofrio BM, Class QA, Rickert ME, Larsson H, Långström N, Lichtenstein P. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry. 2013;70(11):1231-1240. doi: 10.1001/jamapsychiatry.2013.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sucksdorff M, Lehtonen L, Chudal R, et al. . Preterm birth and poor fetal growth as risk factors of attention-deficit/hyperactivity disorder. Pediatrics. 2015;136(3):e599-e608. doi: 10.1542/peds.2015-1043 [DOI] [PubMed] [Google Scholar]
- 13.Nikolas MA, Burt SA. Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis. J Abnorm Psychol. 2010;119(1):1-17. doi: 10.1037/a0018010 [DOI] [PubMed] [Google Scholar]
- 14.Kuntsi J, Pinto R, Price TS, van der Meere JJ, Frazier-Wood AC, Asherson P. The separation of ADHD inattention and hyperactivity-impulsivity symptoms: pathways from genetic effects to cognitive impairments and symptoms. J Abnorm Child Psychol. 2014;42(1):127-136. doi: 10.1007/s10802-013-9771-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greven CU, Rijsdijk FV, Plomin R. A twin study of ADHD symptoms in early adolescence: hyperactivity-impulsivity and inattentiveness show substantial genetic overlap but also genetic specificity. J Abnorm Child Psychol. 2011;39(2):265-275. doi: 10.1007/s10802-010-9451-9 [DOI] [PubMed] [Google Scholar]
- 16.Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997;38(8):931-941. doi: 10.1111/j.1469-7610.1997.tb01612.x [DOI] [PubMed] [Google Scholar]
- 17.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010;49(5):453-63.e1. [PubMed] [Google Scholar]
- 18.Murray E, Pearson R, Fernandes M, et al. . Are fetal growth impairment and preterm birth causally related to child attention problems and ADHD? evidence from a comparison between high-income and middle-income cohorts. J Epidemiol Community Health. 2016;70(7):704-709. doi: 10.1136/jech-2015-206222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shum D, Neulinger K, O’Callaghan M, Mohay H. Attentional problems in children born very preterm or with extremely low birth weight at 7-9 years. Arch Clin Neuropsychol. 2008;23(1):103-112. doi: 10.1016/j.acn.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 20.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490-499. doi: 10.1007/s13311-012-0135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2010;33(2):357-373. doi: 10.1016/j.psc.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 22.Magnus P, Birke C, Vejrup K, et al. . Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016;45(2):382-388. doi: 10.1093/ije/dyw029 [DOI] [PubMed] [Google Scholar]
- 23.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C; MoBa Study Group . Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2006;35(5):1146-1150. doi: 10.1093/ije/dyl170 [DOI] [PubMed] [Google Scholar]
- 24.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):257-268. doi: 10.1023/A:1022602400621 [DOI] [PubMed] [Google Scholar]
- 25.Achenbach TM, Dumenci L, Rescorla LA. DSM-oriented and empirically based approaches to constructing scales from the same item pools. J Clin Child Adolesc Psychol. 2003;32(3):328-340. doi: 10.1207/S15374424JCCP3203_02 [DOI] [PubMed] [Google Scholar]
- 26.Silva RR, Alpert M, Pouget E, et al. . A rating scale for disruptive behavior disorders, based on the DSM-IV item pool. Psychiatr Q. 2005;76(4):327-339. doi: 10.1007/s11126-005-4966-x [DOI] [PubMed] [Google Scholar]
- 27.Skrondal A, Rabe-Hesketh S. Generalized Latent Variable Modeling: Multilevel, Longitudinal, and Structural Equation Models. Boca Raton, FL: CRC Press; 2004. doi: 10.1201/9780203489437 [DOI] [Google Scholar]
- 28.Zambrana IM, Vollrath ME, Sengpiel V, Jacobsson B, Ystrom E. Preterm delivery and risk for early language delays: a sibling-control cohort study. Int J Epidemiol. 2016;45(1):151-159. doi: 10.1093/ije/dyv329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. 2016;21(2):68-73. doi: 10.1016/j.siny.2015.12.011 [DOI] [PubMed] [Google Scholar]
- 30.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75-84. doi: 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843-848. doi: 10.1111/j.1651-2227.1996.tb14164.x [DOI] [PubMed] [Google Scholar]
- 32.Muthén LK, Muthén BO. Mplus User’s Guide . 6th ed. Los Angeles, CA: Muthén & Muthén; 1998-2010. [Google Scholar]
- 33.Enders CK. Applied Missing Data Analysis. New York, NY: Guilford Press; 2010. [Google Scholar]
- 34.Borenstein M, Hedges LV, Higgins J, Rothstein HR. Converting among effect sizes In: Introduction to Meta-analysis. Hoboken, NJ: John Wiley & Sons; 2009:45-49. doi: 10.1002/9780470743386.ch7 [DOI] [Google Scholar]
- 35.Heinonen K, Räikkönen K, Pesonen A-K, et al. . Behavioural symptoms of attention deficit/hyperactivity disorder in preterm and term children born small and appropriate for gestational age: a longitudinal study. BMC Pediatr. 2010;10(1):91. doi: 10.1186/1471-2431-10-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr. 2015;169(12):1162-1172. doi: 10.1001/jamapediatrics.2015.2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farooqi A, Hägglöf B, Sedin G, Gothefors L, Serenius F. Mental health and social competencies of 10- to 12-year-old children born at 23 to 25 weeks of gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics. 2007;120(1):118-133. doi: 10.1542/peds.2006-2988 [DOI] [PubMed] [Google Scholar]
- 38.Perlman JM. Neurobehavioral deficits in premature graduates of intensive care: potential medical and neonatal environmental risk factors. Pediatrics. 2001;108(6):1339-1348. doi: 10.1542/peds.108.6.1339 [DOI] [PubMed] [Google Scholar]
- 39.Nilsen RM, Vollset SE, Gjessing HK, et al. . Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597-608. doi: 10.1111/j.1365-3016.2009.01062.x [DOI] [PubMed] [Google Scholar]
- 40.Frisell T, Öberg S, Kuja-Halkola R, Sjölander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23(5):713-720. doi: 10.1097/EDE.0b013e31825fa230 [DOI] [PubMed] [Google Scholar]
- 41.Sjölander A, Zetterqvist J. Confounders, mediators, or colliders: what types of shared covariates does a sibling comparison design control for? Epidemiology. 2017;28(4):540-547. doi: 10.1097/EDE.0000000000000649 [DOI] [PubMed] [Google Scholar]
- 42.Lubke GH, Hudziak JJ, Derks EM, van Bijsterveldt TC, Boomsma DI. Maternal ratings of attention problems in ADHD: evidence for the existence of a continuum. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1085-1093. doi: 10.1097/CHI.0b013e3181ba3dbb [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Selected Descriptive Characteristics of the MoBa and Sibling Samples
eTable 2. Potential Covariates Associated With Symptoms of ADHD
eTable 3. Potential Covariates Associated With Gestational Week at Birth
eTable 4. Mean Scores of the ADHD Symptom Scales by Gestational Age at Birth
eTable 5. Sex Stratified Mean Scores of the ADHD Symptom Scales by Gestational Week (GW) at Birth
eMethods. Covariate Selection
eFigure. Illustration of the Difference Between Conventional Cohort Analyses (Step 1 and 2) and Sibling Comparison Analyses (Step 3)