This systematic review and meta-analysis assesses the prevalence of postmenopausal bleeding among women with endometrial cancer and the risk of endometrial cancer in women with postmenopausal bleeding.
Key Points
Question
What is the prevalence of postmenopausal bleeding in women with endometrial cancer and the risk of endometrial cancer in women with postmenopausal bleeding?
Findings
This systematic review and meta-analysis of 40 790 unique patients in 129 unique studies suggests that postmenopausal bleeding occurs in approximately 90% of women with endometrial cancer; however, only 9% of women with postmenopausal bleeding were diagnosed with endometrial cancer. These estimates varied by geographic region, hormone use, and calendar time.
Meaning
These findings provide a foundation for evaluating early detection strategies for endometrial cancer and can support risk-informed decision making in clinical management of postmenopausal bleeding.
Abstract
Importance
As the worldwide burden of endometrial cancer continues to rise, interest is growing in the evaluation of early detection and prevention strategies among women at increased risk. Focusing efforts on women with postmenopausal bleeding (PMB), a common symptom of endometrial cancer, may be a useful strategy; however, PMB is not specific for endometrial cancer and is often caused by benign conditions.
Objective
To provide a reference of the prevalence of PMB in endometrial cancers and the risk of endometrial cancer in women with PMB.
Data Sources
For this systematic review and meta-analysis, PubMed and Embase were searched for English-language studies published January 1, 1977, through January 31, 2017.
Study Selection
Observational studies reporting the prevalence of PMB in women with endometrial cancer and the risk of endometrial cancer in women with PMB in unselected populations were selected.
Data Extraction and Synthesis
Two independent reviewers evaluated study quality and risk of bias using items from the Newcastle-Ottawa Quality Assessment Scale and the Quality Assessment of Diagnostic Accuracy Studies tool. Studies that included highly selected populations, lacked detailed inclusion criteria, and/or included 25 or fewer women were excluded.
Main Outcomes and Measures
The pooled prevalence of PMB in women with endometrial cancer and the risk of endometrial cancer in women with PMB.
Results
A total of 129 unique studies, including 34 432 unique patients with PMB and 6358 with endometrial cancer (40 790 women), were analyzed. The pooled prevalence of PMB among women with endometrial cancer was 91% (95% CI, 87%-93%), irrespective of tumor stage. The pooled risk of endometrial cancer among women with PMB was 9% (95% CI, 8%-11%), with estimates varying by use of hormone therapy (range, 7% [95% CI, 6%-9%] to 12% [95% CI, 9%-15%]; P < .001 for heterogeneity) and geographic region (range, 5% [95% CI, 3%-11%] in North America to 13% [95% CI, 9%-19%] in Western Europe; P = .09 for heterogeneity).
Conclusions and Relevance
Early detection strategies focused on women with PMB have the potential to capture as many as 90% of endometrial cancers; however, most women with PMB will not be diagnosed with endometrial cancer. These results can aid in the assessment of the potential clinical value of new early detection markers and clinical management strategies for endometrial cancer and will help to inform clinical and epidemiologic risk prediction models to support decision making.
Introduction
Endometrial cancer is the most common gynecologic cancer in developed countries and accounts for nearly 5% of cancer cases and more than 2% of deaths due to cancer in women worldwide.1 In regions such as North America and parts of Europe, the incidence of endometrial cancer is disproportionately higher than in other developed countries, which may be attributed to higher rates of obesity, as well as other important risk factors such as aging, early menarche, late menopause, nulliparity, and postmenopausal estrogen therapy use.2 Unlike most cancers, the incidence of endometrial cancer and associated mortality rates have increased in recent years3,4,5,6,7 and are projected to rise during the next 10 years.8,9,10,11
Most endometrial cancers are diagnosed at a localized stage and are often curable with surgery, with a 5-year survival of approximately 95%. In contrast, 5-year survival for late-stage (stage IV) endometrial cancer ranges from 16% to 45%.12,13,14 However, studies evaluating early detection strategies for endometrial cancer are lacking, and at present no recommendation for population-based screening exists. In the era of precision prevention, emphasis on identifying individuals at high risk to maximize the positive outcomes of clinical interventions while avoiding unnecessary harms is growing.15,16,17 Rather than targeting the whole population, early detection strategies for endometrial cancer could focus on women at high risk of developing endometrial cancer, while excluding most women at low risk. Postmenopausal bleeding (PMB) is a common symptom of endometrial cancer and accounts for approximately two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.18 Women presenting with PMB undergo additional clinical testing using a combination of transvaginal ultrasonography (TVUS), hysteroscopy, endometrial biopsy, and/or dilation and curettage, and workup varies widely among different settings.18,19,20 However, PMB is often associated with benign conditions such as endometrial polyps or may result from unscheduled bleeding in women using hormone therapy (HT).18,21 The risk of endometrial cancer in women with PMB varies widely in individual studies from 3% to 25%.22,23,24,25,26,27
Accurate estimates of the prevalence of PMB in endometrial cancers (equal to the sensitivity of PMB for detecting endometrial cancer) and the risk of endometrial cancer in women with PMB (equal to the positive predictive value [PPV] of PMB for detecting endometrial cancer) are needed to evaluate whether targeting women with PMB for early detection is a useful strategy, particularly because endometrial cancer rates are increasing in the population. A high sensitivity of PMB would ensure that most cases of endometrial cancer are being captured by targeting this population. A high PPV of PMB, which translates into a low number needed to diagnose (1/PPV) to find 1 case of endometrial cancer, would support diagnostic workup of women with PMB, whereas a low PPV would signify the need for additional triage to improve performance of early detection. Furthermore, these estimates would provide the foundation for evaluating clinical and epidemiologic risk prediction models28 and are necessary for evaluating novel molecular markers that are currently under development against established methods.29,30,31,32,33
We conducted a systematic review and meta-analysis to evaluate the prevalence of PMB in women with endometrial cancer and the risk of endometrial cancer among women with PMB. Our estimates could inform the evaluation of clinical scenarios to assess the utility of early detection strategies for endometrial cancer.
Methods
Search Strategy and Selection Criteria
We conducted this systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (eFigure 1 in the Supplement).34 We included original studies with primary data reporting the prevalence of PMB in women with endometrial cancer and the risk of endometrial cancer among women with PMB. We searched English-language, peer-reviewed studies published before February 1, 2017, in the MEDLINE database via PubMed and Embase using search terms described in eMethods in the Supplement. We also reviewed the reference lists of articles identified in the primary search for additional relevant studies. Titles and abstracts were independently screened for inclusion by 3 investigators (M.A.C., A.D.M., and B.J.L.). Full-text versions of eligible articles were reviewed by 2 investigators (M.A.C. and B.J.L.) to determine eligibility; any questions regarding the inclusion of studies were resolved by the senior author (N.W.). We evaluated data on patient selection criteria, sample size, and exposure and outcome ascertainment to determine study quality and generalizability; we excluded studies that included special populations (eg, defined by comorbid conditions or specific histologic findings), lacked detailed inclusion criteria, and/or included 25 or fewer women.
Data Extraction and Quality Assessment
We extracted information on aggregate study-level participant characteristics (age, body mass index, years since menopause, parity, frequency of bleeding, HT use, tamoxifen use, and other comorbidities) and endometrial biopsy results, including stage and histologic data when available. Geographic regions were defined by the World Health Organization for those with 2 or more countries represented.1 Study designs were classified as retrospective or prospective if follow-up time was specified or as cross-sectional (or case series). We assessed study quality using items from the Newcastle-Ottawa Quality Assessment Scale35 and the Quality Assessment of Diagnostic Accuracy Studies tool36 (eMethods in the Supplement). We provide the detailed algorithms of how PMB was evaluated for each study in eTable 1 in the Supplement. Studies were classified as having potential verification bias if receipt or interpretation of the diagnostic test (eg, endometrial biopsy) depended on the results of a prior clinical test (eg, TVUS) (eMethods in the Supplement).
Data Synthesis and Analysis
We estimated pooled prevalence and 95% CIs using multilevel logistic-normal random-effects models to account for interstudy heterogeneity. Between-study variance was quantified using the τ2 statistic.37,38 We visualized variation in study-specific estimates using forest plots and performed subgroup analyses (described in eMethods in the Supplement) to evaluate the influence of (1) study exclusion criteria for HT use (analysis of risk of endometrial cancer in women with PMB only); (2) geographical region; and (3) study enrollment period, using the last year of study enrollment or publication date as a proxy, grouped as before 1990, 1990 to 1999, 2000 to 2009, and 2010 to 2017. We use the P value for heterogeneity to compare subgroup estimates, with significance at P < .05. The influence of continuous study-level (mean) characteristics, including age, years since menopause, and percentage using HT, was explored using multilevel logistic random-effects models for studies with available data. We conducted sensitivity analyses to assess the influence of clinical setting (tertiary center vs other), study design, and the potential for publication bias using Egger regression analyses.29 For the analysis of the prevalence of PMB in women with endometrial cancer, we excluded 2 studies39,40 that selected cases based on stage at diagnosis; however, these studies were included in the stage-specific analysis. For the analysis of the risk of endometrial cancer among women with PMB, we conducted a secondary analysis in a subset of 10 studies41,42,43,44,45,46,47,48,49,50 that excluded women with measurements below a minimum endometrial thickness determined by TVUS (range, 4-5 mm) and a separate subset of 7 studies51,52,53,54,55,56,57 that evaluated the risk of endometrial cancer in women with polyps.
As an ancillary analysis, we simulated the performance of 2 approaches for early detection of endometrial cancer in a hypothetical population of 10 000 women with PMB to demonstrate how our results can be used to evaluate current testing strategies and the potential clinical value of early-stage biomarkers for endometrial cancer detection: TVUS (cutoff of ≤3 mm), which is a well-established, clinically validated test,18 and an experimental methylation marker assay31,32 (eMethods in the Supplement). All analyses were performed in Stata, version 13 (StataCorp). For pooling of proportions, we used the program metaprop_one.38
Results
We identified 2398 studies, of which 129 were eligible for our analysis,22,23,24,25,26,27,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162 with 40 790 unique patients, including 1 study58 that was eligible for both analyses (overlap of 45 women with endometrial cancers and 45 women with PMB) (eFigure 1 in the Supplement). Studies were published from January 1, 1977, through January 1, 2017, and most were cross-sectional and conducted in Northern (26 [20.2%]) and Southern Europe (24 [18.6%]). Among eligible studies, 2139,40,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,74,75,76,77 were included for analysis of the prevalence of PMB in women with endometrial cancer (3792 cases of endometrial cancer, of which 3257 were in women with PMB, including the 2 studies restricted to stages III-IV cancers39,40) and 9222,23,24,25,26,27,58,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162 were included for analysis of the risk of endometrial cancer in women with PMB (31 220 women with PMB and 2611 cases of endometrial cancer).
Prevalence of PMB in Women With Endometrial Cancer
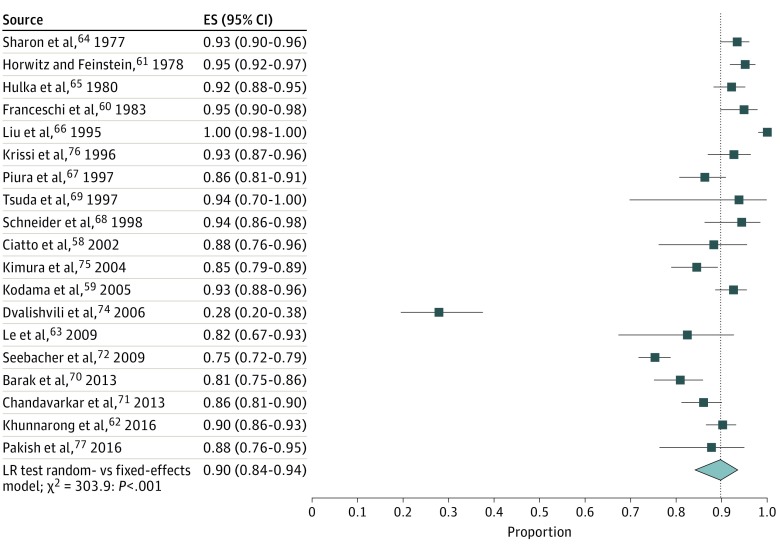
Study-specific and pooled estimates of the prevalence of PMB in women with endometrial cancer are shown in Figure 1. The prevalence of PMB was 90% (95% CI, 84%-94%), with substantial between-study variance (τ2 = 1.14). Removal of a potential outlier74 resulted in a similar pooled prevalence of 91% (95% CI, 87%-93%), but strong reduction of variance between studies (τ2 = 0.47); therefore we excluded the outlier study from the remaining analyses. Among 5 studies66,70,72,75,76 with information on stage I tumors, the proportion of PMB was 94% (95% CI, 72%-99%; τ2 = 4.03). Among the 7 studies39,40,66,70,72,75,76 with information on stages II to IV tumors, the proportion of PMB was 84% (95% CI, 71%-92%; τ2 = 0.93). We found no significant difference in prevalence of PMB by stage (P = .20 for heterogeneity) (eFigure 2 in the Supplement).
Figure 1. Prevalence of Postmenopausal Bleeding (PMB) in Women With Endometrial Cancer.
The pooled prevalence of PMB is indicated by the dotted line. ES indicates effect size; LR, likelihood ratio; and diamond, pooled risk.
In an analysis stratified by geographic region, the prevalence of PMB ranged from 94% (95% CI, 84%-97%) in North America to 90% in Western Asia (95% CI, 85%-94%) and Eastern Asia (95% CI, 83%-94%) (P = .55 for heterogeneity) (eFigure 3 in the Supplement). The pooled prevalence of PMB among women with endometrial cancer varied significantly by study enrollment period (P < .001 for heterogeneity). The prevalence of PMB in women with endometrial cancer was higher in studies that enrolled women before 1990 (94%; 95% CI, 92%-95%) and in 1990 to 1999 (96%; 95% CI, 87%-99%) compared with studies that enrolled women in 2000 to 2009 (85%; 95% CI, 78%-90%) and in 2010 to 2017 (86%; 95% CI, 82%-90%) (eFigure 4 in the Supplement).
In a sensitivity analysis restricted to 11 studies59,62,64,66,67,68,70,71,72,75,76 (67%) that ascertained PMB through retrospective medical record review, the pooled prevalence of PMB was 91% (95% CI, 85%-94%), similar to our overall findings. The prevalence of PMB did not vary significantly by clinical setting. No evidence of publication bias was found among studies reporting the prevalence of PMB in women with endometrial cancer (Egger regression intercept, 0.15; P = .90).
Risk of Endometrial Cancer in Women With PMB
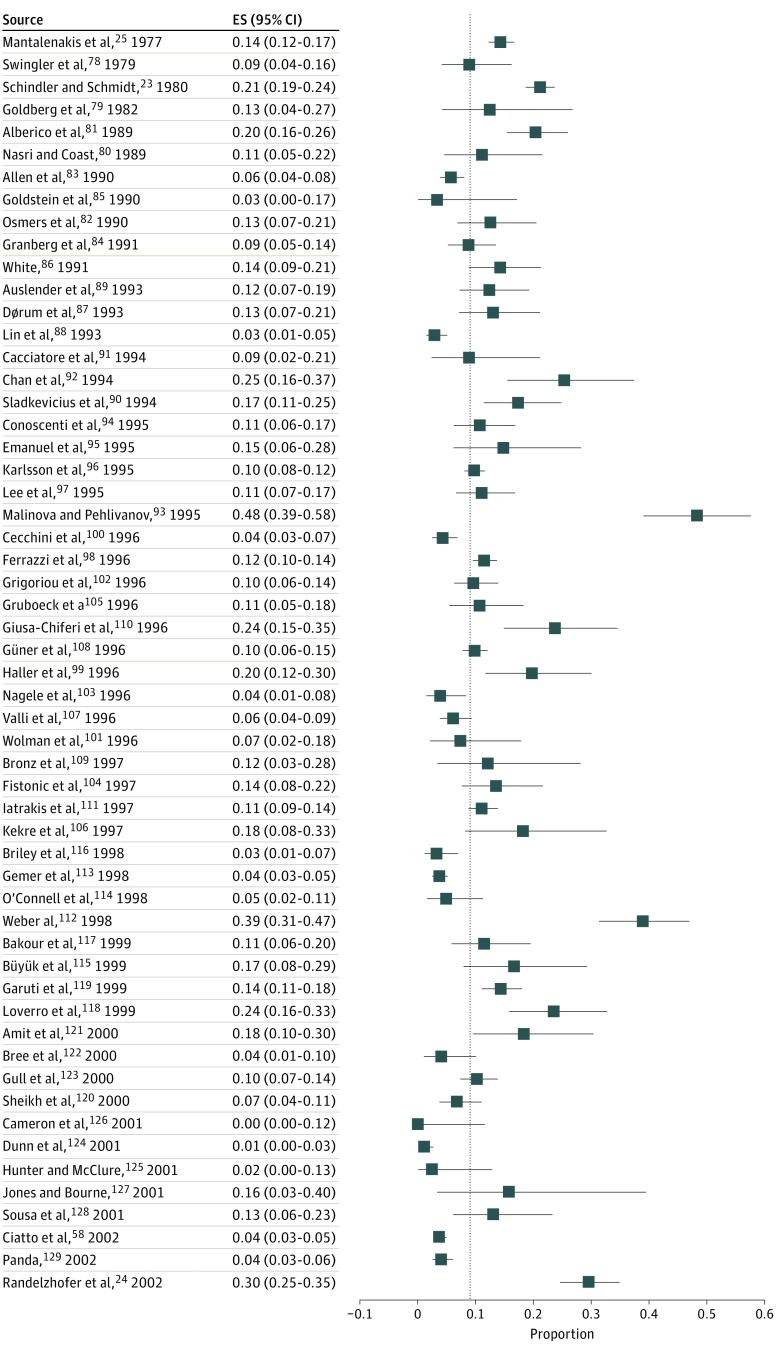
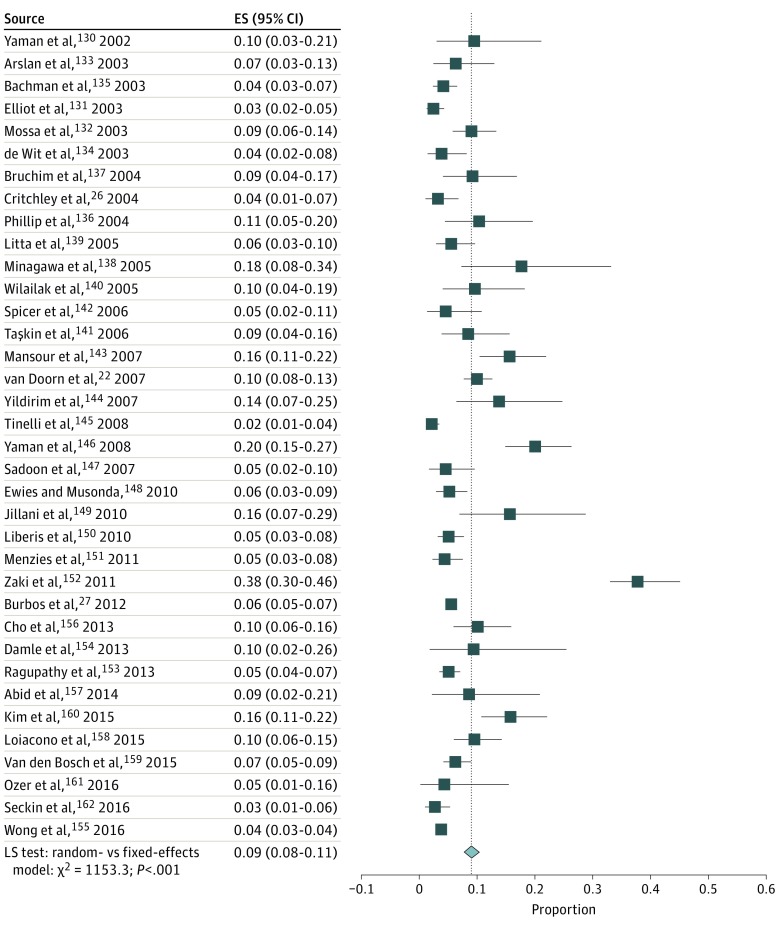
Study-specific and pooled estimates of the risk of endometrial cancer in women with PMB are shown in Figures 2 and 3. In 92 studies,22,23,24,25,26,27,58,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162 the risk of endometrial cancer ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%), with moderate variability observed between studies (τ2 = 0.56).
Figure 2. Risk of Endometrial Cancer in Women With Postmenopausal Bleeding.
The pooled risk of endometrial cancer in all 92 studies is indicated by the dotted line. ES indicates effect size.
Figure 3. Risk of Endometrial Cancer in Women With Postmenopausal Bleeding.
Figure 3 is a continuation of Figure 2. The pooled risk of endometrial cancer in all 92 studies is indicated by the dotted line. ES indicates effect size; LR, likelihood ratio; and diamond, pooled risk.
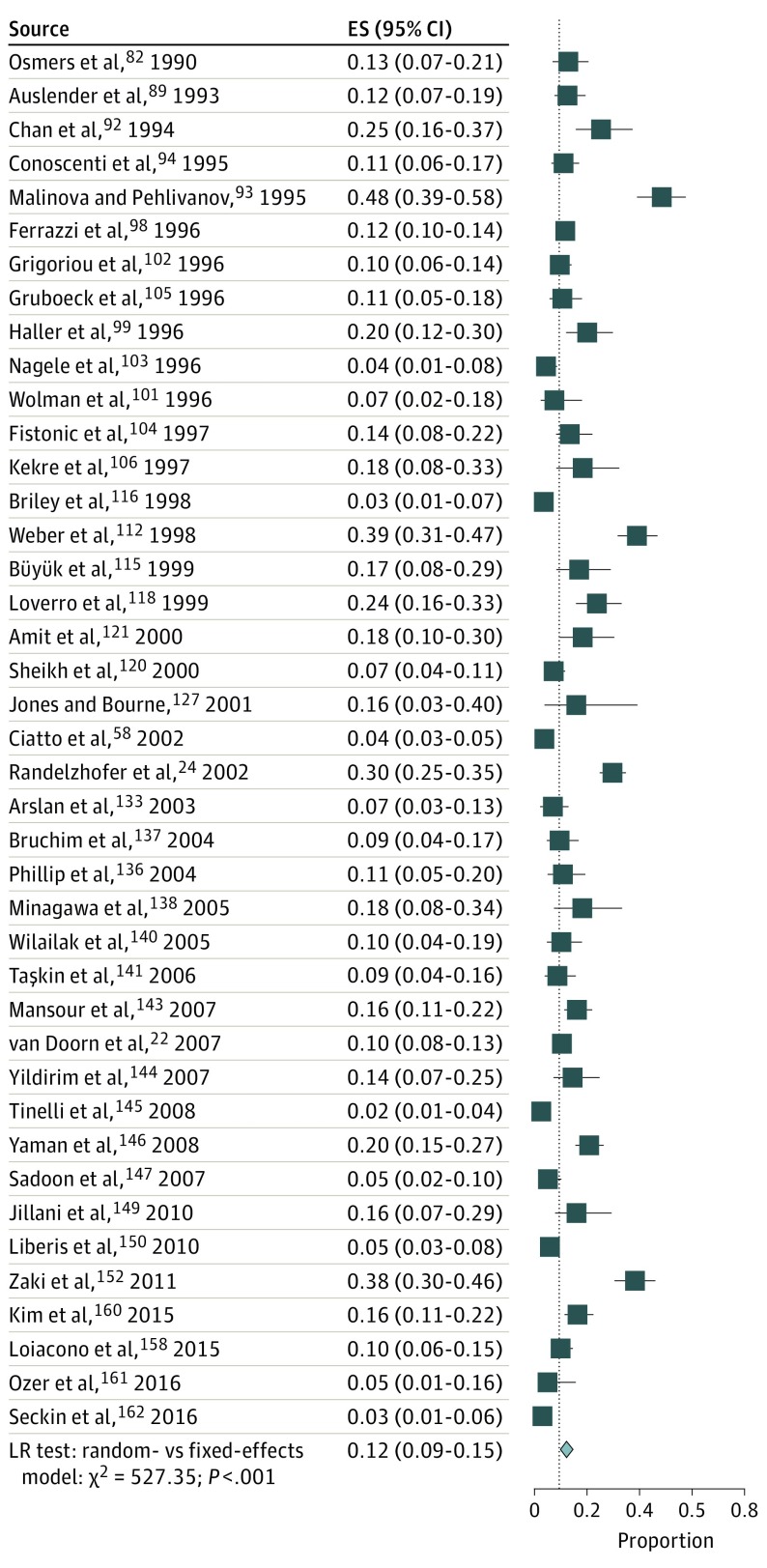
The pooled risk of endometrial cancer was significantly higher among the 41 studies22,24,58,82,89,92,93,94,98,99,101,102,103,104,105,106,112,115,116,118,120,121,127,133,136,137,138,140,141,143,144,145,146,147,149,150,152,158,160,161,162 that excluded women using HT (12%; 95% CI, 9%-15%; τ2 = 0.64) (Figure 4) compared with the 51 studies that included women using HT21,23,25,26,27,78,79,80,81,83,84,85,86,87,88,90,91,95,96,97,100,107,108,109,110,111,113,114,117,119,122,123,124,125,126,128,129,130,131,134,135,139,142,148,151,153,154,155,156,157,159 (7%; 95% CI, 6%-9%; τ2 = 0.38; P < .001 for heterogeneity) (eFigure 8 in the Supplement).
Figure 4. Risk of Endometrial Cancer in Women With Postmenopausal Bleeding in Studies That Excluded Women Using HT.
The pooled risk of endometrial cancer in all 92 studies is indicated by the dotted line. ES indicates effect size; HT, hormone therapy; LR, likelihood ratio; and diamond, pooled risk.
The risk of endometrial cancer in women with PMB was lowest in North America (5%; 95% CI, 3%-11%; τ2 = 0.78) and Northern Europe (7%; 95% CI, 5%-8%; τ2 = 0.24) and highest in Western Europe (13%; 95% CI, 9%-19%; τ2 = 0.61) (P = .09 for heterogeneity) (eFigure 5 in the Supplement). In an analysis restricted to European countries only, the risk of endometrial cancer was significantly higher in Western Europe compared with Northern and Southern Europe (P = .03 for heterogeneity). After stratifying by exclusion of women who used HT, significant regional differences persisted in both strata (P = .02 for heterogeneity in studies that included women using HT; P < .001 for heterogeneity in studies that excluded women using HT).
The risk of endometrial cancer was significantly higher in studies with enrollment periods before 1990 (13%; 95% CI, 10%-17%; τ2 = 0.18) and in 1990 to 1999 (11%; 95% CI, 8%-13%; τ2 = 0.57) compared with 2000 to 2009 (7%; 95% CI, 5%-9%; τ2 = 0.47) and 2010 to 2017 (8%; 95% CI, 5%-12%; τ2 = 0.59) (P < .001 for heterogeneity) (eFigure 6 in the Supplement). The risk of endometrial cancer was not significantly associated with mean age, number of years since menopause, and percentage of women using HT.
The risk of endometrial cancer was significantly lower in prospective22,26,27,58,88,105,122,123,124,135,138,139,140,150 (6%; τ2 = 0.34) and retrospective23,25,83,94,97,113,125,131,134,147,148,151,153,155,158,161 (6%; τ2 = 0.37) studies compared with cross-sectional studies78,79,80,81,82,84,85,86,87,90,91,92,93,95,96,98,99,100,101,102,103,104,106,107,108,109,110,111,112,114,115,116,117,118,119,120,121,126,127,128,129,130,132,133,136,137,141,142,143,144,145,146,149,152,154,156,157,159,160,162 (11%; τ2 = 0.49; P < .001 for heterogeneity) and was significantly higher in 6 studies24,110,121,130,152,157 conducted in tertiary centers (23%; 95% CI, 17%-31%; τ2 = 0.18; P < .001 for heterogeneity), compared with studies conducted in other settings. Evidence of publication bias suggested that small studies may overestimate the risk of endometrial cancer in women with PMB (Egger regression intercept, 0.75; P = .001). In an analysis based on the assessment of study quality, verification bias could be excluded in 71 studies24,25,26,58,78,79,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,98,99,100,101,102,103,104,105,106,108,110,111,112,113,114,115,117,118,119,120,121,122,125,126,128,130,131,132,133,135,136,137,139,140,141,143,144,145,146,147,149,150,152,154,156,157,158,161,162 and was potentially present in 13 studies22,27,107,109,123,124,127,129,138,142,148,155,159 (8 were unclear). The risk of endometrial cancer was significantly lower in studies with potential verification bias (6%; 95% CI, 4%-9%) compared with those with no verification bias (10%; 95% CI, 8%-12%) (eTable 2 and eFigure 7 in the Supplement).
In the 10 studies41,42,43,44,45,46,47,48,49,50 that included women with PMB and a minimum endometrial thickness (n = 2087), the pooled risk of endometrial cancer was 19% (95% CI, 14%-25%; τ2 = 0.28). In 7 studies51,52,53,54,55,56,57 restricted to women with PMB and polyps (n = 2801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%; τ2 = 0).
To demonstrate how the estimates from this meta-analysis can be used to evaluate strategies for endometrial cancer detection in women with PMB, we evaluated the performance of TVUS, a well-established clinical test for evaluating PMB18 and an experimental methylation assay for endometrial cancer detection31,32 in a hypothetical population of 10 000 women with PMB. We evaluated endometrial cancer risk estimates of 5%, 10%, and 15%, representing the range of risks observed in different geographic regions (Table). We show the magnitude of the increase in PPV of both tests with increasing risk of endometrial cancer in women with PMB, supporting the evaluation of early-detection strategies in various populations.
Table. Clinical Performance of Transvaginal Ultrasonography and an Experimental Assay for Endometrial Cancer Detection Across Risk Estimates in a Hypothetical Population of 10 000 Women With PMBa.
| Endometrial Cancer Risk in PMB, % | Test Sensitivity, % | Test Specificity, % | PPV, % | cNPV, % | No. of Patients With Biopsy | No. of Biopsies per case of Cancer |
|---|---|---|---|---|---|---|
| 5 | ||||||
| Transvaginal ultrasonography | 98.0 | 35.0 | 7.4 | 0.3 | 6665 | 13.6 |
| Methylation assay | 90.0 | 50.0 | 8.7 | 1.0 | 5200 | 11.6 |
| 10 | ||||||
| Transvaginal ultrasonography | 98.0 | 35.0 | 14.3 | 0.3 | 6830 | 7.0 |
| Methylation assay | 90.0 | 50.0 | 16.7 | 2.2 | 5400 | 6.0 |
| 15 | ||||||
| Transvaginal ultrasonography | 98.0 | 35.0 | 21.0 | 1.0 | 6995 | 4.8 |
| Methylation assay | 90.0 | 50.0 | 24.1 | 3.4 | 5600 | 4.1 |
Abbreviations: cNPV, complement of the negative predictive value; PMB, postmenopausal bleeding; PPV, positive predictive value.
Assumes 10 000 women with PMB; sensitivity and specificity estimates for transvaginal ultrasonography and methylation were obtained from the literature.
Discussion
The projected rise in endometrial cancer incidence and mortality underscores the importance of strategies for early detection and prevention. Focusing on women at highest risk of endometrial cancer can greatly improve the performance of a diagnostic test and avoid unnecessary testing and associated harms among women at low risk. Our systematic review and meta-analysis demonstrates that PMB is very sensitive for endometrial cancer detection, occurring in approximately 90% of cases. However, our findings indicate that among women with PMB, only approximately 9% will be diagnosed with endometrial cancer, with estimates varying substantially by HT use, geographic region, and the presence of endometrial polyps. Current practice guidelines recommend workup to rule out endometrial cancer among all women with PMB. Our findings support this recommendation by providing reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most endometrial cancers. However, the low PPV of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and avoid unnecessary biopsies in low-risk women.
The prevalence of PMB in endometrial cancer and the risk of endometrial cancer in women with PMB were higher before 2000 compared with after 2000. When interpreting these results, it is important to distinguish population risk, which has generally increased over time, from the risk in women with PMB. The number of endometrial cancers without PMB and the number of women with PMB with benign conditions may both have increased over time. This increase could be influenced by factors such as changes in HT use, changes in prevalence of obesity, or changes in clinical management thresholds for abnormal bleeding.
The risk of endometrial cancer among women with PMB was notably lower in studies that included HT users compared with those that excluded these women. Use of HT may affect this association at multiple levels. Certain combined formulations of estrogen plus progestin therapy are established to have a protective effect on the endometrium.163 Furthermore, irregular uterine bleeding is a common adverse effect of HT, particularly within the first 6 months of use.164 The underlying causes of HT-induced bleeding is thought to involve changes in the size of endometrial blood vessels and regulation of vascular growth and integrity.165 Because this type of bleeding is generally not associated with abnormal endometrial histologic findings, most guidelines recommend against clinical workup of women using HT who experience irregular uterine bleeding within the first 6 months. However, little consensus exists about how to best treat these women if bleeding persists, and a considerable number of women with HT-associated bleeding will undergo procedures to rule out endometrial cancer.165 Our data emphasize the importance of considering a woman’s HT status to inform clinical decision making, potentially supporting a less aggressive management approach in HT users.
We noted striking geographic differences in endometrial cancer risk among women with PMB, ranging from 13% in Western Europe to 5% in North America and 7% in Northern Europe. At present, consensus regarding the optimal approach for evaluating PMB is lacking. Practice may vary depending on resources, clinical expertise and judgment, and patient preferences. The threshold for evaluating PMB may be lower in North American countries compared with other countries in Europe and elsewhere. In many European countries, guidelines recommend TVUS as the first-line test, with histologic assessment indicated for women with a thickened endometrium based on cutoffs ranging from 3 to 5 mm.18,166,167 In the United States, guidelines recommend TVUS or endometrial biopsy as the first step in evaluating PMB.19 In sensitivity analyses, we observed a lower risk of endometrial cancer in studies with partial disease verification (ie, not all women received a biopsy) compared with studies with complete diagnostic verification, suggesting that disease may have been missed in women with negative findings for the first-line test (eg, TVUS). However, we cannot exclude that in some settings, women only received a first-line test such as TVUS if they had a lower risk of endometrial cancer. In the subset of studies included in our meta-analysis that included women with PMB and a minimum endometrial thickness, the pooled risk of endometrial cancer was 19%, more than double the risk observed in our main analysis.
Our findings also suggest substantial variation in the risk of endometrial cancer depending on the underlying cause of PMB. Endometrial polyps are one of the most common causes of PMB. Although polyps have been associated with risk of endometrial cancer in women with PMB,168 other studies have suggested that this association is more likely attributed to detection bias, resulting from incidental findings during the diagnostic workup of PMB caused by endometrial polyps.169 Our meta-analysis confirms a lower risk of endometrial cancer among women with PMB and polyps.
Strengths and Limitations
To our knowledge, this systematic review and meta-analysis is the first to evaluate the prevalence of PMB in endometrial cancer and the risk of endometrial cancer in women with PMB, 2 important variables for evaluating the role of PMB in early detection of endometrial cancer. Our findings can support risk-informed decision making in clinical management of women with PMB. As an example, we simulated the performance of TVUS, an established diagnostic tool, and methylation markers, an early-phase biomarker, for early detection of endometrial cancer. We provided estimates of how many women would be referred for endometrial biopsy for combinations of endometrial cancer risk in women with PMB, and we showed how many women would need to undergo endometrial biopsy to identify 1 case.
However, a few study limitations are worth noting. In general, data on study-level variables such as years since menopause and body mass index were inconsistently reported, limiting our ability to evaluate them. In addition, insufficient data were available to explore differences by histologic findings, stage, and grade. Whether cancers with more favorable histologic findings (eg, endometrioid type I tumors) are more likely to present with PMB compared with more aggressive histologic subtypes (eg, serous type II tumors) remains unknown. Our results suggest that approximately 10% of women diagnosed with endometrial cancer do not present with PMB. Given the cross-sectional nature of most studies included in this meta-analysis, additional studies linking clinical records with cancer registry data may be warranted to validate our findings. With respect to the analysis of the risk of endometrial cancer in women with PMB, most studies were cross-sectional, and few included prospective follow-up; thus, we were unable to evaluate long-term risk of endometrial cancer in these studies. Finally, our results suggested a lower prevalence of endometrial cancer in retrospective and prospective cohort studies compared with cross-sectional studies. Cross-sectional studies may have been more likely to include women with recurrent bleeding; however, few studies distinguished between incident vs recurrent PMB.
Conclusions
The widespread practice of referring all women with PMB for TVUS and/or endometrial biopsy carries a considerable burden and cost. Given the rise in endometrial cancer incidence and mortality, our findings raise the important question of how to best manage PMB to optimize the benefit of early detection approaches while avoiding unnecessary harms. Interest has increased in the use of biomarkers, such as DNA methylation, to improve early detection of endometrial cancer.31,32,170,171 To obtain reliable estimates of the clinical performance of molecular assays, diagnostic tests, and management algorithms, we must know the prior risk of endometrial cancer in the population.29,172
Our study represents an important and timely evaluation of the risk of endometrial cancer in women with PMB and can serve as a reliable reference for the prevalence of PMB in women with endometrial cancer and the risk of endometrial cancer in women with PMB, 2 requisite prior probabilities for prediction of endometrial cancer risk and secondary and tertiary prevention. As new markers are discovered or new clinical management strategies are evaluated, our results can aid in the assessment of their potential clinical value and will help to inform clinical and epidemiologic risk prediction models to support clinical decision making.
eFigure 1. PRISMA Flow Diagram
eFigure 2. Prevalence of PMB in Women With Stage I vs Stages II-IV Endometrial Cancers
eFigure 3. Prevalence of PMB in Women With Endometrial Cancer, Stratified by Geographic Region
eFigure 4. Prevalence of PMB in Women With Endometrial Cancer by Study Enrollment Period
eFigure 5. Risk of Endometrial Cancer in Women With PMB by Geographic Region
eFigure 6. Risk of Endometrial Cancer in Women With PMB, Stratified by Study Enrollment Period
eFigure 7. Risk of Endometrial Cancer in Women With PMB by Potential for Study Verification Bias
eFigure 8. Risk of Endometrial Cancer in Women With Postmenopausal Bleeding (PMB) by Hormone Therapy Selection Criteria
eMethods. Study Retrieval and Evaluation and Data Analysis
eTable 1. Results of Quality Assessment of the 92 Studies Included in the Analysis of Risk of Endometrial Cancer in Women With PMB
eTable 2. Results of Sensitivity Analyses Based on Quality Assessment
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. . Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Setiawan VW, Yang HP, Pike MC, et al. ; Australian National Endometrial Cancer Study Group . Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31(20):2607-2618. doi: 10.1200/JCO.2012.48.2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamison PM, Noone AM, Ries LA, Lee NC, Edwards BK. Trends in endometrial cancer incidence by race and histology with a correction for the prevalence of hysterectomy, SEER 1992 to 2008. Cancer Epidemiol Biomarkers Prev. 2013;22(2):233-241. doi: 10.1158/1055-9965.EPI-12-0996 [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev. 2017;26(4):444-457. doi: 10.1158/1055-9965.EPI-16-0858 [DOI] [PubMed] [Google Scholar]
- 5.Wartko P, Sherman ME, Yang HP, Felix AS, Brinton LA, Trabert B. Recent changes in endometrial cancer trends among menopausal-age US women. Cancer Epidemiol. 2013;37(4):374-377. doi: 10.1016/j.canep.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 7.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst. 2018;110(4):354-361. doi: 10.1093/jnci/djx214 [DOI] [PubMed] [Google Scholar]
- 8.Lindemann K, Eskild A, Vatten LJ, Bray F. Endometrial cancer incidence trends in Norway during 1953-2007 and predictions for 2008-2027. Int J Cancer. 2010;127(11):2661-2668. doi: 10.1002/ijc.25267 [DOI] [PubMed] [Google Scholar]
- 9.Gaber C, Meza R, Ruterbusch JJ, Cote ML. Endometrial cancer trends by race and histology in the USA: projecting the number of new cases from 2015 to 2040. J Racial Ethn Health Disparities. Published online October 17, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheikh MA, Althouse AD, Freese KE, et al. . USA endometrial cancer projections to 2030: should we be concerned? Future Oncol. 2014;10(16):2561-2568. doi: 10.2217/fon.14.192 [DOI] [PubMed] [Google Scholar]
- 11.Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115(9):1147-1155. doi: 10.1038/bjc.2016.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiderpass E, Antoine J, Bray FI, Oh JK, Arbyn M. Trends in corpus uteri cancer mortality in member states of the European Union. Eur J Cancer. 2014;50(9):1675-1684. doi: 10.1016/j.ejca.2014.02.020 [DOI] [PubMed] [Google Scholar]
- 13.Creasman WT, Odicino F, Maisonneuve P, et al. . Carcinoma of the corpus uteri: FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(suppl 1):S105-S143. doi: 10.1016/S0020-7292(06)60031-3 [DOI] [PubMed] [Google Scholar]
- 14.UK CR Uterine cancer statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer. Updated July 3, 2017. Accessed January 18, 2018.
- 15.Kitson SJ, Evans DG, Crosbie EJ. Identifying high-risk women for endometrial cancer prevention strategies: proposal of an endometrial cancer risk prediction model. Cancer Prev Res (Phila). 2017;10(1):1-13. doi: 10.1158/1940-6207.CAPR-16-0224 [DOI] [PubMed] [Google Scholar]
- 16.Castle PE, Katki HA. Screening: a risk-based framework to decide who benefits from screening. Nat Rev Clin Oncol. 2016;13(9):531-532. doi: 10.1038/nrclinonc.2016.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girschik J, Miller LJ, Addiscott T, et al. . Precision in setting cancer prevention priorities: synthesis of data, literature, and expert opinion. Front Public Health. 2017;5:125. doi: 10.3389/fpubh.2017.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Hanegem N, Breijer MC, Khan KS, et al. . Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68(2):155-164. doi: 10.1016/j.maturitas.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 19.ACOG Committee opinion No. 734: The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129. doi: 10.1097/AOG.0000000000002631 [DOI] [PubMed] [Google Scholar]
- 20.Breijer MC, Timmermans A, van Doorn HC, Mol BW, Opmeer BC. Diagnostic strategies for postmenopausal bleeding. Obstet Gynecol Int. 2010;2010:850812. doi: 10.1155/2010/850812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burbos N, Musonda P, Duncan TJ, Crocker SG, Nieto JJ, Morris EP. Postmenopausal vaginal bleeding in women using hormone replacement therapy. Menopause Int. 2012;18(1):5-9. doi: 10.1258/mi.2011.011111 [DOI] [PubMed] [Google Scholar]
- 22.van Doorn HC, Opmeer BC, Jitze Duk M, Kruitwagen RF, Dijkhuizen FP, Mol BW. The relation between age, time since menopause, and endometrial cancer in women with postmenopausal bleeding. Int J Gynecol Cancer. 2007;17(5):1118-1123. doi: 10.1111/j.1525-1438.2007.00925.x [DOI] [PubMed] [Google Scholar]
- 23.Schindler AE, Schmidt G. Post-menopausal bleeding: a study of more than 1000 cases. Maturitas. 1980;2(4):269-274. doi: 10.1016/0378-5122(80)90028-6 [DOI] [PubMed] [Google Scholar]
- 24.Randelzhofer B, Prömpeler HJ, Sauerbrei W, Madjar H, Emons G. Value of sonomorphological criteria of the endometrium in women with postmenopausal bleeding: a multivariate analysis. Ultrasound Obstet Gynecol. 2002;19(1):62-68. doi: 10.1046/j.0960-7692.2001.00618.x [DOI] [PubMed] [Google Scholar]
- 25.Mantalenakis SJ, Papapostolou MG. Genital bleeding in women aged 50 and over. Int Surg. 1977;62(2):103-105. [PubMed] [Google Scholar]
- 26.Critchley HO, Warner P, Lee AJ, Brechin S, Guise J, Graham B. Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status. Health Technol Assess. 2004;8(34):iii-iv, 1-139. doi: 10.3310/hta8340 [DOI] [PubMed] [Google Scholar]
- 27.Burbos N, Musonda P, Crocker SG, Morris EP, Nieto JJ, Duncan TJ. Management of postmenopausal women with vaginal bleeding when the endometrium can not be visualized. Acta Obstet Gynecol Scand. 2012;91(6):686-691. doi: 10.1111/j.1600-0412.2012.01407.x [DOI] [PubMed] [Google Scholar]
- 28.van Hanegem N, Breijer MC, Opmeer BC, Mol BW, Timmermans A. Prediction models in women with postmenopausal bleeding: a systematic review. Womens Health (Lond). 2012;8(3):251-262. doi: 10.2217/WHE.12.10 [DOI] [PubMed] [Google Scholar]
- 29.Wentzensen N, Wacholder S. From differences in means between cases and controls to risk stratification: a business plan for biomarker development. Cancer Discov. 2013;3(2):148-157. doi: 10.1158/2159-8290.CD-12-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talhouk A, McConechy MK, Leung S, et al. . Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123(5):802-813. doi: 10.1002/cncr.30496 [DOI] [PubMed] [Google Scholar]
- 31.Wentzensen N, Bakkum-Gamez JN, Killian JK, et al. . Discovery and validation of methylation markers for endometrial cancer. Int J Cancer. 2014;135(8):1860-1868. doi: 10.1002/ijc.28843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakkum-Gamez JN, Wentzensen N, Maurer MJ, et al. . Detection of endometrial cancer via molecular analysis of DNA collected with vaginal tampons. Gynecol Oncol. 2015;137(1):14-22. doi: 10.1016/j.ygyno.2015.01.552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stelloo E, Bosse T, Nout RA, et al. . Refining prognosis and identifying targetable pathways for high-risk endometrial cancer: a TransPORTEC initiative. Mod Pathol. 2015;28(6):836-844. doi: 10.1038/modpathol.2015.43 [DOI] [PubMed] [Google Scholar]
- 34.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 35.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf. 2014. Accessed July 2017.
- 36.Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137-159. doi: 10.1111/j.1467-985X.2008.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackillop WJ, Pringle JF. Stage III endometrial carcinoma: a review of 90 cases. Cancer. 1985;56(10):2519-2523. doi: [DOI] [PubMed] [Google Scholar]
- 40.Pliskow S, Penalver M, Averette HE. Stage III and stage IV endometrial carcinoma: a review of 41 cases. Gynecol Oncol. 1990;38(2):210-215. doi: 10.1016/0090-8258(90)90043-K [DOI] [PubMed] [Google Scholar]
- 41.Williams SC, Lopez C, Yoong A, McHugo JM. Developing a robust and efficient pathway for the referral and investigation of women with post-menopausal bleeding using a cut-off of < or =4 mm for normal thickness. Br J Radiol. 2007;80(957):719-723. doi: 10.1259/bjr/87219886 [DOI] [PubMed] [Google Scholar]
- 42.de Kroon CD, Hiemstra E, Trimbos JB, Jansen FW. Power Doppler area in the diagnosis of endometrial cancer. Int J Gynecol Cancer. 2010;20(7):1160-1165. doi: 10.1111/IGC.0b013e3181f0df98 [DOI] [PubMed] [Google Scholar]
- 43.Alcazar JL, Galvan R. Three-dimensional power Doppler ultrasound scanning for the prediction of endometrial cancer in women with postmenopausal bleeding and thickened endometrium. Am J Obstet Gynecol. 2009;200(1):44.e1-44.e6. doi: 10.1016/j.ajog.2008.08.027 [DOI] [PubMed] [Google Scholar]
- 44.Opolskiene G, Sladkevicius P, Jokubkiene L, Valentin L. Three-dimensional ultrasound imaging for discrimination between benign and malignant endometrium in women with postmenopausal bleeding and sonographic endometrial thickness of at least 4.5 mm. Ultrasound Obstet Gynecol. 2010;35(1):94-102. doi: 10.1002/uog.7445 [DOI] [PubMed] [Google Scholar]
- 45.Elfayomy AK, El Tarhouny SA. Ovarian volume assessment in relation to histologic findings and sex hormone levels in women with postmenopausal bleeding and thickened endometrium. Ann Saudi Med. 2012;32(6):588-592. doi: 10.5144/0256-4947.2012.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi A, Forzano L, Romanello I, Fachechi G, Marchesoni D. Assessment of endometrial volume and vascularization using transvaginal 3D power Doppler angiography in women with postmenopausal bleeding. Int J Gynaecol Obstet. 2012;119(1):14-17. doi: 10.1016/j.ijgo.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 47.Makled AK, Elmekkawi SF, El-Refaie TA, El-Sherbiny MA. Three-dimensional power Doppler and endometrial volume as predictors of malignancy in patients with postmenopausal bleeding. J Obstet Gynaecol Res. 2013;39(5):1045-1051. doi: 10.1111/j.1447-0756.2012.02066.x [DOI] [PubMed] [Google Scholar]
- 48.Salman MC, Bozdag G, Dogan S, Yuce K. Role of postmenopausal bleeding pattern and women’s age in the prediction of endometrial cancer. Aust N Z J Obstet Gynaecol. 2013;53(5):484-488. [DOI] [PubMed] [Google Scholar]
- 49.Dueholm M, Møller C, Rydbjerg S, Hansen ES, Ørtoft G. An ultrasound algorithm for identification of endometrial cancer. Ultrasound Obstet Gynecol. 2014;43(5):557-568. doi: 10.1002/uog.13205 [DOI] [PubMed] [Google Scholar]
- 50.Giannella L, Mfuta K, Setti T, Cerami LB, Bergamini E, Boselli F. A risk-scoring model for the prediction of endometrial cancer among symptomatic postmenopausal women with endometrial thickness > 4 mm. Biomed Res Int. 2014;2014:130569. doi: 10.1155/2014/130569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernández-Parra J, Rodríguez Oliver A, López Criado S, Parrilla Fernández F, Montoya Ventoso F. Hysteroscopic evaluation of endometrial polyps. Int J Gynaecol Obstet. 2006;95(2):144-148. doi: 10.1016/j.ijgo.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 52.Domingues AP, Lopes H, Dias I, De Oliveira CF. Endometrial polyps in postmenopausal women. Acta Obstet Gynecol Scand. 2009;88(5):618-620. doi: 10.1080/00016340902818188 [DOI] [PubMed] [Google Scholar]
- 53.Golan A, Cohen-Sahar B, Keidar R, Condrea A, Ginath S, Sagiv R. Endometrial polyps: symptomatology, menopausal status and malignancy. Gynecol Obstet Invest. 2010;70(2):107-112. doi: 10.1159/000298767 [DOI] [PubMed] [Google Scholar]
- 54.Mossa B, Torcia F, Avenoso F, Tucci S, Marziani R. Occurrence of malignancy in endometrial polyps during postmenopause. Eur J Gynaecol Oncol. 2010;31(2):165-168. [PubMed] [Google Scholar]
- 55.Ronghe R, Gaudoin M. Women with recurrent postmenopausal bleeding should be re-investigated but are not more likely to have endometrial cancer. Menopause Int. 2010;16(1):9-11. doi: 10.1258/mi.2010.010008 [DOI] [PubMed] [Google Scholar]
- 56.Wethington SL, Herzog TJ, Burke WM, et al. . Risk and predictors of malignancy in women with endometrial polyps. Ann Surg Oncol. 2011;18(13):3819-3823. doi: 10.1245/s10434-011-1815-z [DOI] [PubMed] [Google Scholar]
- 57.Cavkaytar S, Kokanali MK, Ceran U, Topcu HO, Sirvan L, Doganay M. Roles of sonography and hysteroscopy in the detection of premalignant and malignant polyps in women presenting with postmenopausal bleeding and thickened endometrium. Asian Pac J Cancer Prev. 2014;15(13):5355-5358. doi: 10.7314/APJCP.2014.15.13.5355 [DOI] [PubMed] [Google Scholar]
- 58.Ciatto S, Cecchini S, Gervasi G, Landini A, Zappa M, Crocetti E. Association of endometrial thickness assessed at trans-vaginal ultrasonography to endometrial cancer in postmenopausal women asymptomatic or with abnormal uterine bleeding. Radiol Med. 2002;104(5-6):437-442. [PubMed] [Google Scholar]
- 59.Kodama J, Seki N, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y. Correlation of presenting symptoms and patient characteristics with endometrial cancer prognosis in Japanese women. Int J Gynaecol Obstet. 2005;91(2):151-156. doi: 10.1016/j.ijgo.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 60.Franceschi S, La Vecchia C, Gallus G, et al. . Delayed diagnosis of endometrial cancer in Italy. Cancer. 1983;51(6):1176-1178. doi: [DOI] [PubMed] [Google Scholar]
- 61.Horwitz RI, Feinstein AR. Alternative analytic methods for case-control studies of estrogens and endometrial cancer. N Engl J Med. 1978;299(20):1089-1094. doi: 10.1056/NEJM197811162992001 [DOI] [PubMed] [Google Scholar]
- 62.Khunnarong J, Tangjitgamol S, Srijaipracharoen S. Other gynecologic pathology in endometrial cancer patients. Asian Pac J Cancer Prev. 2016;17(2):713-717. doi: 10.7314/APJCP.2016.17.2.713 [DOI] [PubMed] [Google Scholar]
- 63.Le T, Menard C, Samant R, et al. . Longitudinal assessments of quality of life in endometrial cancer patients: effect of surgical approach and adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(3):795-802. doi: 10.1016/j.ijrobp.2008.11.018 [DOI] [PubMed] [Google Scholar]
- 64.Sharon Z, Shani M, Modan B. Clinicoepidemiologic study of uterine cancer: comparative aspects of the endometrial and cervical sites. Obstet Gynecol. 1977;50(5):536-540. [PubMed] [Google Scholar]
- 65.Hulka BS, Grimson RC, Greenberg BG, et al. . “Alternative” controls in a case-control study of endometrial cancer and exogenous estrogen. Am J Epidemiol. 1980;112(3):376-387. doi: 10.1093/oxfordjournals.aje.a113003 [DOI] [PubMed] [Google Scholar]
- 66.Liu JR, Conaway M, Rodriguez GC, Soper JT, Clarke-Pearson DL, Berchuck A. Relationship between race and interval to treatment in endometrial cancer. Obstet Gynecol. 1995;86(4, pt 1):486-490. doi: 10.1016/0029-7844(95)00238-M [DOI] [PubMed] [Google Scholar]
- 67.Piura B, Bar-Dayan A, Cohen Y, Yanai-Inbar I, Glezerman M. Endometrial carcinoma in the south of Israel: study of 231 cases. J Surg Oncol. 1997;66(3):189-195. doi: [DOI] [PubMed] [Google Scholar]
- 68.Schneider D, Halperin R, Langer R, Bukovsky I, Hermann A. Well-differentiated versus less-differentiated endometrial carcinoma. Eur J Gynaecol Oncol. 1998;19(3):242-245. [PubMed] [Google Scholar]
- 69.Tsuda H, Kawabata M, Yamamoto K, Inoue T, Umesaki N. Prospective study to compare endometrial cytology and transvaginal ultrasonography for identification of endometrial malignancies. Gynecol Oncol. 1997;65(3):383-386. doi: 10.1006/gyno.1997.4699 [DOI] [PubMed] [Google Scholar]
- 70.Barak F, Kalichman L, Gdalevich M, et al. . The influence of early diagnosis of endometrioid endometrial cancer on disease stage and survival. Arch Gynecol Obstet. 2013;288(6):1361-1364. doi: 10.1007/s00404-013-2898-5 [DOI] [PubMed] [Google Scholar]
- 71.Chandavarkar U, Kuperman JM, Muderspach LI, Opper N, Felix JC, Roman L. Endometrial echo complex thickness in postmenopausal endometrial cancer. Gynecol Oncol. 2013;131(1):109-112. doi: 10.1016/j.ygyno.2013.07.109 [DOI] [PubMed] [Google Scholar]
- 72.Seebacher V, Schmid M, Polterauer S, et al. . The presence of postmenopausal bleeding as prognostic parameter in patients with endometrial cancer: a retrospective multi-center study. BMC Cancer. 2009;9:460. doi: 10.1186/1471-2407-9-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Wieslander C, Hansen G, Cass I, Vasilev S, Holschneider CH. Thin endometrial echo complex on ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol. 2006;101(1):120-125. doi: 10.1016/j.ygyno.2005.09.042 [DOI] [PubMed] [Google Scholar]
- 74.Dvalishvili I, Charkviani L, Turashvili G, Burkadze G. Clinical characteristics of prognostic factors in uterine endometrioid adenocarcinoma of various grade. Georgian Med News. 2006;(132):24-27. [PubMed] [Google Scholar]
- 75.Kimura T, Kamiura S, Yamamoto T, Seino-Noda H, Ohira H, Saji F. Abnormal uterine bleeding and prognosis of endometrial cancer. Int J Gynaecol Obstet. 2004;85(2):145-150. doi: 10.1016/j.ijgo.2003.12.001 [DOI] [PubMed] [Google Scholar]
- 76.Krissi H, Chetrit A, Menczer J. Presenting symptoms of patients with endometrial carcinoma: effect on prognosis. Eur J Gynaecol Oncol. 1996;17(1):25-28. [PubMed] [Google Scholar]
- 77.Pakish JB, Lu KH, Sun CC, et al. . Endometrial cancer associated symptoms: a case-control study. J Womens Health (Larchmt). 2016;25(11):1187-1192. doi: 10.1089/jwh.2015.5657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swingler GR, Cave DG, Mitchard P. Diagnostic accuracy of the MiMark endometrial cell sampler in 101 patients with postmenopausal bleeding. Br J Obstet Gynaecol. 1979;86(10):816-818. doi: 10.1111/j.1471-0528.1979.tb10699.x [DOI] [PubMed] [Google Scholar]
- 79.Goldberg GL, Tsalacopoulos G, Davey DA. A comparison of endometrial sampling with the Accurette and Vabra aspirator and uterine curettage. S Afr Med J. 1982;61(4):114-116. [PubMed] [Google Scholar]
- 80.Nasri MN, Coast GJ. Correlation of ultrasound findings and endometrial histopathology in postmenopausal women. Br J Obstet Gynaecol. 1989;96(11):1333-1338. doi: 10.1111/j.1471-0528.1989.tb03233.x [DOI] [PubMed] [Google Scholar]
- 81.Alberico S, Conoscenti G, Vegliò P, Bogatti P, Di Bonito L, Mandruzzato G. A clinical and epidemiological study of 245 postmenopausal metrorrhagia patients. Clin Exp Obstet Gynecol. 1989;16(4):113-121. [PubMed] [Google Scholar]
- 82.Osmers R, Völksen M, Schauer A. Vaginosonography for early detection of endometrial carcinoma? Lancet. 1990;335(8705):1569-1571. doi: 10.1016/0140-6736(90)91387-P [DOI] [PubMed] [Google Scholar]
- 83.Allen DG, Correy JF, Marsden DE. Abnormal uterine bleeding and cancer of the genital tract. Aust N Z J Obstet Gynaecol. 1990;30(1):81-83. doi: 10.1111/j.1479-828X.1990.tb03204.x [DOI] [PubMed] [Google Scholar]
- 84.Granberg S, Wikland M, Karlsson B, Norström A, Friberg LG. Endometrial thickness as measured by endovaginal ultrasonography for identifying endometrial abnormality. Am J Obstet Gynecol. 1991;164(1 Pt 1):47-52. doi: 10.1016/0002-9378(91)90622-X [DOI] [PubMed] [Google Scholar]
- 85.Goldstein SR, Nachtigall M, Snyder JR, Nachtigall L. Endometrial assessment by vaginal ultrasonography before endometrial sampling in patients with postmenopausal bleeding. Am J Obstet Gynecol. 1990;163(1, pt 1):119-123. doi: 10.1016/S0002-9378(11)90683-8 [DOI] [PubMed] [Google Scholar]
- 86.White CD. Post menopausal bleeding as a risk factor for endometrial carcinoma. W V Med J. 1991;87(1):15-17. [PubMed] [Google Scholar]
- 87.Dørum A, Kristensen GB, Langebrekke A, Sørnes T, Skaar O. Evaluation of endometrial thickness measured by endovaginal ultrasound in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 1993;72(2):116-119. doi: 10.3109/00016349309023423 [DOI] [PubMed] [Google Scholar]
- 88.Lin HH, Wu MY, Shyu MK, Chen D, Tsai JL, Hsieh CY. Clinical study of 381 postmenopausal bleeding patients. J Formos Med Assoc. 1993;92(3):241-244. [PubMed] [Google Scholar]
- 89.Auslender R, Bornstein J, Dirnfeld M, Kogan O, Atad J, Abramovici H. Vaginal ultrasonography in patients with postmenopausal bleeding. Ultrasound Obstet Gynecol. 1993;3(6):426-428. doi: 10.1046/j.1469-0705.1993.03060426.x [DOI] [PubMed] [Google Scholar]
- 90.Sladkevicius P, Valentin L, Marsál K. Endometrial thickness and Doppler velocimetry of the uterine arteries as discriminators of endometrial status in women with postmenopausal bleeding: a comparative study. Am J Obstet Gynecol. 1994;171(3):722-728. doi: 10.1016/0002-9378(94)90088-4 [DOI] [PubMed] [Google Scholar]
- 91.Cacciatore B, Ramsay T, Lehtovirta P, Ylöstalo P. Transvaginal sonography and hysteroscopy in postmenopausal bleeding. Acta Obstet Gynecol Scand. 1994;73(5):413-416. doi: 10.3109/00016349409006254 [DOI] [PubMed] [Google Scholar]
- 92.Chan FY, Chau MT, Pun TC, et al. . Limitations of transvaginal sonography and color Doppler imaging in the differentiation of endometrial carcinoma from benign lesions. J Ultrasound Med. 1994;13(8):623-628. doi: 10.7863/jum.1994.13.8.623 [DOI] [PubMed] [Google Scholar]
- 93.Malinova M, Pehlivanov B. Transvaginal sonography and endometrial thickness in patients with postmenopausal uterine bleeding. Eur J Obstet Gynecol Reprod Biol. 1995;58(2):161-165. doi: 10.1016/0028-2243(95)80017-M [DOI] [PubMed] [Google Scholar]
- 94.Conoscenti G, Meir YJ, Fischer-Tamaro L, et al. . Endometrial assessment by transvaginal sonography and histological findings after D & C in women with postmenopausal bleeding. Ultrasound Obstet Gynecol. 1995;6(2):108-115. doi: 10.1046/j.1469-0705.1995.06020108.x [DOI] [PubMed] [Google Scholar]
- 95.Emanuel MH, Verdel MJC, Stas H, Wamsteker K, Lammes FB. An audit of true prevalence of intra-uterine pathology: the hysteroscopical findings controlled for patient selection in 1202 patients with abnormal uterine bleeding. Gynaecol Endosc. 1995;4(4):237-241. [Google Scholar]
- 96.Karlsson B, Granberg S, Wikland M, et al. . Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding: a Nordic multicenter study. Am J Obstet Gynecol. 1995;172(5):1488-1494. doi: 10.1016/0002-9378(95)90483-2 [DOI] [PubMed] [Google Scholar]
- 97.Lee WH, Tan KH, Lee YW. The aetiology of postmenopausal bleeding—a study of 163 consecutive cases in Singapore. Singapore Med J. 1995;36(2):164-168. [PubMed] [Google Scholar]
- 98.Ferrazzi E, Torri V, Trio D, Zannoni E, Filiberto S, Dordoni D. Sonographic endometrial thickness: a useful test to predict atrophy in patients with postmenopausal bleeding: an Italian multicenter study. Ultrasound Obstet Gynecol. 1996;7(5):315-321. doi: 10.1046/j.1469-0705.1996.07050315.x [DOI] [PubMed] [Google Scholar]
- 99.Haller H, Matecjcić N, Rukavina B, Krasević M, Rupcić S, Mozetic D. Transvaginal sonography and hysteroscopy in women with postmenopausal bleeding. Int J Gynaecol Obstet. 1996;54(2):155-159. doi: 10.1016/0020-7292(96)02677-X [DOI] [PubMed] [Google Scholar]
- 100.Cecchini S, Ciatto S, Bonardi R, Grazzini G, Mazzota A. Endometrial ultrasonography: an alternative to invasive assessment in women with postmenopausal vaginal bleeding. Tumori. 1996;82(1):38-39. doi: 10.1177/030089169608200107 [DOI] [PubMed] [Google Scholar]
- 101.Wolman I, Sagi J, Ginat S, Jaffa AJ, Hartoov J, Jedwab G. The sensitivity and specificity of vaginal sonography in detecting endometrial abnormalities in women with postmenopausal bleeding. J Clin Ultrasound. 1996;24(2):79-82. doi: [DOI] [PubMed] [Google Scholar]
- 102.Grigoriou O, Kalovidouros A, Papadias C, Antoniou G, Antonaki V, Giannikos L. Transvaginal sonography of the endometrium in women with postmenopausal bleeding. Maturitas. 1996;23(1):9-14. doi: 10.1016/0378-5122(95)00945-0 [DOI] [PubMed] [Google Scholar]
- 103.Nagele F, O’Connor H, Baskett TF, Davies A, Mohammed H, Magos AL. Hysteroscopy in women with abnormal uterine bleeding on hormone replacement therapy: a comparison with postmenopausal bleeding. Fertil Steril. 1996;65(6):1145-1150. doi: 10.1016/S0015-0282(16)58329-0 [DOI] [PubMed] [Google Scholar]
- 104.Fistonic I, Hodek B, Klaric P, Jokanovic L, Grubisic G, Ivicevic-Bakulic T. Transvaginal sonographic assessment of premalignant and malignant changes in the endometrium in postmenopausal bleeding. J Clin Ultrasound. 1997;25(8):431-435. doi: [DOI] [PubMed] [Google Scholar]
- 105.Gruboeck K, Jurkovic D, Lawton F, Savvas M, Tailor A, Campbell S. The diagnostic value of endometrial thickness and volume measurements by three-dimensional ultrasound in patients with postmenopausal bleeding. Ultrasound Obstet Gynecol. 1996;8(4):272-276. doi: 10.1046/j.1469-0705.1996.08040272.x [DOI] [PubMed] [Google Scholar]
- 106.Kekre AN, Jose R, Seshadri L. Transvaginal sonography of the endometrium in south Indian postmenopausal women. Aust N Z J Obstet Gynaecol. 1997;37(4):449-451. doi: 10.1111/j.1479-828X.1997.tb02458.x [DOI] [PubMed] [Google Scholar]
- 107.Valli E, Zupi E, Marconi D, et al. . Vaginal ultrasonography and diagnostic hysteroscopy for women with abnormal uterine bleeding after menopause. J Am Assoc Gynecol Laparosc. 1996;3(4)(suppl):S52. [DOI] [PubMed] [Google Scholar]
- 108.Güner H, Tiras MB, Karabacak O, Sarikaya H, Erdem M, Yildirim M. Endometrial assessment by vaginal ultrasonography might reduce endometrial sampling in patients with postmenopausal bleeding: a prospective study. Aust N Z J Obstet Gynaecol. 1996;36(2):175-178. doi: 10.1111/j.1479-828X.1996.tb03280.x [DOI] [PubMed] [Google Scholar]
- 109.Bronz L, Suter T, Rusca T. The value of transvaginal sonography with and without saline instillation in the diagnosis of uterine pathology in pre- and postmenopausal women with abnormal bleeding or suspect sonographic findings. Ultrasound Obstet Gynecol. 1997;9(1):53-58. doi: 10.1046/j.1469-0705.1997.09010053.x [DOI] [PubMed] [Google Scholar]
- 110.Giusa-Chiferi MG, Gonçalves WJ, Baracat EC, de Albuquerque Neto LC, Bortoletto CC, de Lima GR. Transvaginal ultrasound, uterine biopsy and hysteroscopy for postmenopausal bleeding. Int J Gynaecol Obstet. 1996;55(1):39-44. doi: 10.1016/0020-7292(96)02720-8 [DOI] [PubMed] [Google Scholar]
- 111.Iatrakis G, Diakakis I, Kourounis G, et al. . Postmenopausal uterine bleeding. Clin Exp Obstet Gynecol. 1997;24(3):157. [PubMed] [Google Scholar]
- 112.Weber G, Merz E, Bahlmann F, Rösch B. Evaluation of different transvaginal sonographic diagnostic parameters in women with postmenopausal bleeding. Ultrasound Obstet Gynecol. 1998;12(4):265-270. doi: 10.1046/j.1469-0705.1998.12040265.x [DOI] [PubMed] [Google Scholar]
- 113.Gemer O, Segal S. Endometrial cancer in patients undergoing diagnostic curettage. Arch Gynecol Obstet. 1998;261(2):79-81. doi: 10.1007/s004040050203 [DOI] [PubMed] [Google Scholar]
- 114.O’Connell LP, Fries MH, Zeringue E, Brehm W. Triage of abnormal postmenopausal bleeding: a comparison of endometrial biopsy and transvaginal sonohysterography versus fractional curettage with hysteroscopy. Am J Obstet Gynecol. 1998;178(5):956-961. doi: 10.1016/S0002-9378(98)70530-7 [DOI] [PubMed] [Google Scholar]
- 115.Büyük E, Durmuşoğlu F, Erenus M, Karakoç B. Endometrial disease diagnosed by transvaginal ultrasound and dilatation and curettage. Acta Obstet Gynecol Scand. 1999;78(5):419-422. doi: 10.1080/j.1600-0412.1999.780514.x [DOI] [PubMed] [Google Scholar]
- 116.Briley M, Lindsell DR. The role of transvaginal ultrasound in the investigation of women with post-menopausal bleeding. Clin Radiol. 1998;53(7):502-505. doi: 10.1016/S0009-9260(98)80169-4 [DOI] [PubMed] [Google Scholar]
- 117.Bakour SH, Dwarakanath LS, Khan KS, Newton JR, Gupta JK. The diagnostic accuracy of ultrasound scan in predicting endometrial hyperplasia and cancer in postmenopausal bleeding. Acta Obstet Gynecol Scand. 1999;78(5):447-451. doi: 10.1080/j.1600-0412.1999.780519.x [DOI] [PubMed] [Google Scholar]
- 118.Loverro G, Bettocchi S, Cormio G, et al. . Transvaginal sonography and hysteroscopy in postmenopausal uterine bleeding. Maturitas. 1999;33(2):139-144. doi: 10.1016/S0378-5122(99)00023-7 [DOI] [PubMed] [Google Scholar]
- 119.Garuti G, Sambruni I, Cellani F, Garzia D, Alleva P, Luerti M. Hysteroscopy and transvaginal ultrasonography in postmenopausal women with uterine bleeding. Int J Gynaecol Obstet. 1999;65(1):25-33. doi: 10.1016/S0020-7292(98)00224-0 [DOI] [PubMed] [Google Scholar]
- 120.Sheikh M, Sawhney S, Khurana A, Al-Yatama M. Alteration of sonographic texture of the endometrium in post-menopausal bleeding: a guide to further management. Acta Obstet Gynecol Scand. 2000;79(11):1006-1010. [PubMed] [Google Scholar]
- 121.Amit A, Weiner Z, Ganem N, et al. . The diagnostic value of power Doppler measurements in the endometrium of women with postmenopausal bleeding. Gynecol Oncol. 2000;77(2):243-247. doi: 10.1006/gyno.2000.5766 [DOI] [PubMed] [Google Scholar]
- 122.Bree RL, Bowerman RA, Bohm-Velez M, et al. . US evaluation of the uterus in patients with postmenopausal bleeding: a positive effect on diagnostic decision making. Radiology. 2000;216(1):260-264. doi: 10.1148/radiology.216.1.r00jl37260 [DOI] [PubMed] [Google Scholar]
- 123.Gull B, Carlsson S, Karlsson B, Ylöstalo P, Milsom I, Granberg S. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding: is it always necessary to perform an endometrial biopsy? Am J Obstet Gynecol. 2000;182(3):509-515. doi: 10.1067/mob.2000.103092 [DOI] [PubMed] [Google Scholar]
- 124.Dunn TS, Stamm CA, Delorit M, Goldberg G. Clinical pathway for evaluating women with abnormal uterine bleeding. J Reprod Med. 2001;46(9):831-834. [PubMed] [Google Scholar]
- 125.Hunter DC, McClure N. Abnormal uterine bleeding: an evaluation endometrial biopsy, vaginal ultrasound and outpatient hysteroscopy. Ulster Med J. 2001;70(1):25-30. [PMC free article] [PubMed] [Google Scholar]
- 126.Cameron ST, Walker J, Chambers S, Critchley H. Comparison of transvaginal ultrasound, saline infusion sonography and hysteroscopy to investigate postmenopausal bleeding and unscheduled bleeding on HRT. Aust N Z J Obstet Gynaecol. 2001;41(3):291-294. doi: 10.1111/j.1479-828X.2001.tb01230.x [DOI] [PubMed] [Google Scholar]
- 127.Jones K, Bourne T. The feasibility of a “one stop” ultrasound-based clinic for the diagnosis and management of abnormal uterine bleeding. Ultrasound Obstet Gynecol. 2001;17(6):517-521. doi: 10.1046/j.1469-0705.2001.00445.x [DOI] [PubMed] [Google Scholar]
- 128.Sousa R, Silvestre M, Almeida e Sousa L, et al. . Transvaginal ultrasonography and hysteroscopy in postmenopausal bleeding: a prospective study. Acta Obstet Gynecol Scand. 2001;80(9):856-862. [PubMed] [Google Scholar]
- 129.Panda JK. One-stop clinic for postmenopausal bleeding. J Reprod Med. 2002;47(9):761-766. [PubMed] [Google Scholar]
- 130.Yaman C, Ebner T, Jesacher K, Obermayr G, Pölz W, Tews G. Reproducibility of three-dimensional ultrasound endometrial volume measurements in patients with postmenopausal bleeding. Ultrasound Obstet Gynecol. 2002;19(3):282-286. doi: 10.1046/j.1469-0705.2002.00644.x [DOI] [PubMed] [Google Scholar]
- 131.Elliott J, Connor ME, Lashen H. The value of outpatient hysteroscopy in diagnosing endometrial pathology in postmenopausal women with and without hormone replacement therapy. Acta Obstet Gynecol Scand. 2003;82(12):1112-1119. doi: 10.1046/j.1600-0412.2003.00261.x [DOI] [PubMed] [Google Scholar]
- 132.Mossa B, Imperato F, Marziani R, et al. . Hormonal replacement therapy and evaluation of intrauterine pathology in postmenopausal women: a ten-year study. Eur J Gynaecol Oncol. 2003;24(6):507-512. [PubMed] [Google Scholar]
- 133.Arslan M, Erdem A, Erdem M, Yazici G, Himmetoglu O, Gursoy R. Transvaginal color Doppler ultrasonography for prediction of pre-cancerous endometrial lesions. Int J Gynaecol Obstet. 2003;80(3):299-306. doi: 10.1016/S0020-7292(02)00374-0 [DOI] [PubMed] [Google Scholar]
- 134.de Wit AC, Vleugels MP, de Kruif JH. Diagnostic hysteroscopy: a valuable diagnostic tool in the diagnosis of structural intra-cavital pathology and endometrial hyperplasia or carcinoma? six years of experience with non-clinical diagnostic hysteroscopy. Eur J Obstet Gynecol Reprod Biol. 2003;110(1):79-82. doi: 10.1016/S0301-2115(03)00165-9 [DOI] [PubMed] [Google Scholar]
- 135.Bachmann LM, ter Riet G, Clark TJ, Gupta JK, Khan KS. Probability analysis for diagnosis of endometrial hyperplasia and cancer in postmenopausal bleeding: an approach for a rational diagnostic workup. Acta Obstet Gynecol Scand. 2003;82(6):564-569. doi: 10.1034/j.1600-0412.2003.00176.x [DOI] [PubMed] [Google Scholar]
- 136.Phillip H, Dacosta V, Fletcher H, Kulkarni S, Reid M. Correlation between transvaginal ultrasound measured endometrial thickness and histopathological findings in Afro-Caribbean Jamaican women with postmenopausal bleeding. J Obstet Gynaecol. 2004;24(5):568-572. doi: 10.1080/01443610410001722671 [DOI] [PubMed] [Google Scholar]
- 137.Bruchim I, Biron-Shental T, Altaras MM, et al. . Combination of endometrial thickness and time since menopause in predicting endometrial cancer in women with postmenopausal bleeding. J Clin Ultrasound. 2004;32(5):219-224. doi: 10.1002/jcu.20020 [DOI] [PubMed] [Google Scholar]
- 138.Minagawa Y, Sato S, Ito M, Onohara Y, Nakamoto S, Kigawa J. Transvaginal ultrasonography and endometrial cytology as a diagnostic schema for endometrial cancer. Gynecol Obstet Invest. 2005;59(3):149-154. doi: 10.1159/000083089 [DOI] [PubMed] [Google Scholar]
- 139.Litta P, Merlin F, Saccardi C, et al. . Role of hysteroscopy with endometrial biopsy to rule out endometrial cancer in postmenopausal women with abnormal uterine bleeding. Maturitas. 2005;50(2):117-123. doi: 10.1016/j.maturitas.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 140.Wilailak S, Jirapinyo M, Theppisai U. Transvaginal Doppler sonography: is there a role for this modality in the evaluation of women with postmenopausal bleeding? Maturitas. 2005;50(2):111-116. doi: 10.1016/j.maturitas.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 141.Taşkin S, Bozaci EA, Seval MM, Unlü C. Transvaginal sonographic assessment of endometrial thickness and endometrial morphology in postmenopausal bleeding. Int J Gynaecol Obstet. 2006;92(2):155-156. doi: 10.1016/j.ijgo.2005.10.023 [DOI] [PubMed] [Google Scholar]
- 142.Spicer JM, Siebert I, Kruger TF. Postmenopausal bleeding: a diagnostic approach for both private and public sectors. Gynecol Obstet Invest. 2006;61(3):174-178. doi: 10.1159/000091413 [DOI] [PubMed] [Google Scholar]
- 143.Mansour GM, El-Lamie IK, El-Kady MA, El-Mekkawi SF, Laban M, Abou-Gabal AI. Endometrial volume as predictor of malignancy in women with postmenopausal bleeding. Int J Gynaecol Obstet. 2007;99(3):206-210. doi: 10.1016/j.ijgo.2007.07.024 [DOI] [PubMed] [Google Scholar]
- 144.Yildirim M, Bozkurt N, Kurdoglu M, Taskiran C, Oktem M, Dilek KU. Histopathologic findings in women with postmenopausal bleeding: implication for endometrial thickness and circulating levels of sex steroid hormones. Arch Gynecol Obstet. 2007;276(4):305-310. doi: 10.1007/s00404-007-0361-1 [DOI] [PubMed] [Google Scholar]
- 145.Tinelli R, Tinelli FG, Cicinelli E, Malvasi A, Tinelli A. The role of hysteroscopy with eye-directed biopsy in postmenopausal women with uterine bleeding and endometrial atrophy. Menopause. 2008;15(4, pt 1):737-742. doi: 10.1097/gme.0b013e31815b644e [DOI] [PubMed] [Google Scholar]
- 146.Yaman C, Habelsberger A, Tews G, Pölz W, Ebner T. The role of three-dimensional volume measurement in diagnosing endometrial cancer in patients with postmenopausal bleeding. Gynecol Oncol. 2008;110(3):390-395. doi: 10.1016/j.ygyno.2008.04.029 [DOI] [PubMed] [Google Scholar]
- 147.Sadoon S, Salman G, Smith G, Henson C, McCullough W. Ultrasonographic endometrial thickness for diagnosing endometrial pathology in postmenopausal bleeding. J Obstet Gynaecol. 2007;27(4):406-408. doi: 10.1080/01443610701327438 [DOI] [PubMed] [Google Scholar]
- 148.Ewies AA, Musonda P. Managing postmenopausal bleeding revisited: what is the best first line investigation and who should be seen within 2 weeks? a cross-sectional study of 326 women. Eur J Obstet Gynecol Reprod Biol. 2010;153(1):67-71. doi: 10.1016/j.ejogrb.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 149.Jillani K, Khero RB, Maqsood S, Siddiqui MA. Prevalence of malignant disorders in 50 cases of postmenopausal bleeding. J Pak Med Assoc. 2010;60(7):540-543. [PubMed] [Google Scholar]
- 150.Liberis V, Tsikouras P, Christos Z, et al. . The contribution of hysteroscopy to the detection malignancy in symptomatic postmenopausal women. Minim Invasive Ther Allied Technol. 2010;19(2):83-93. doi: 10.3109/13645701003643881 [DOI] [PubMed] [Google Scholar]
- 151.Menzies R, Wallace S, Ennis M, et al. . Significance of abnormal sonographic findings in postmenopausal women with and without bleeding. J Obstet Gynaecol Can. 2011;33(9):944-951. doi: 10.1016/S1701-2163(16)35020-4 [DOI] [PubMed] [Google Scholar]
- 152.Zaki A, Gaber A, Ghanem E, Moemen M, Shehata G. Abdominal obesity and endometrial cancer in Egyptian females with postmenopausal bleeding. Nutr Cancer. 2011;63(8):1272-1278. doi: 10.1080/01635581.2011.615973 [DOI] [PubMed] [Google Scholar]
- 153.Ragupathy K, Cawley N, Ridout A, Iqbal P, Alloub M. Non-assessable endometrium in women with post-menopausal bleeding: to investigate or ignore. Arch Gynecol Obstet. 2013;288(2):375-378. doi: 10.1007/s00404-013-2746-7 [DOI] [PubMed] [Google Scholar]
- 154.Damle RP, Dravid NV, Suryawanshi KH, Gadre AS, Bagale PS, Ahire N. Clinicopathological spectrum of endometrial changes in peri-menopausal and post-menopausal abnormal uterine bleeding: a 2 years study. J Clin Diagn Res. 2013;7(12):2774-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wong AS, Lao TT, Cheung CW, et al. . Reappraisal of endometrial thickness for the detection of endometrial cancer in postmenopausal bleeding: a retrospective cohort study. BJOG. 2016;123(3):439-446. doi: 10.1111/1471-0528.13342 [DOI] [PubMed] [Google Scholar]
- 156.Cho HJ, Lee ES, Lee JY, et al. . Investigations for postmenopausal uterine bleeding: special considerations for endometrial volume. Arch Iran Med. 2013;16(11):665-670. [PubMed] [Google Scholar]
- 157.Abid M, Hashmi AA, Malik B, et al. . Clinical pattern and spectrum of endometrial pathologies in patients with abnormal uterine bleeding in Pakistan: need to adopt a more conservative approach to treatment. BMC Womens Health. 2014;14:132. doi: 10.1186/s12905-014-0132-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Loiacono RM, Trojano G, Del Gaudio N, et al. . Hysteroscopy as a valid tool for endometrial pathology in patients with postmenopausal bleeding or asymptomatic patients with a thickened endometrium: hysteroscopic and histological results. Gynecol Obstet Invest. 2015;79(3):210-216. doi: 10.1159/000371758 [DOI] [PubMed] [Google Scholar]
- 159.Van den Bosch T, Ameye L, Van Schoubroeck D, Bourne T, Timmerman D. Intra-cavitary uterine pathology in women with abnormal uterine bleeding: a prospective study of 1220 women. Facts Views Vis Obgyn. 2015;7(1):17-24. [PMC free article] [PubMed] [Google Scholar]
- 160.Kim A, Lee JY, Chun S, Kim HY. Diagnostic utility of three-dimensional power Doppler ultrasound for postmenopausal bleeding. Taiwan J Obstet Gynecol. 2015;54(3):221-226. doi: 10.1016/j.tjog.2013.10.043 [DOI] [PubMed] [Google Scholar]
- 161.Ozer A, Ozer S, Kanat-Pektas M. Correlation between transvaginal ultrasound measured endometrial thickness and histopathological findings in Turkish women with abnormal uterine bleeding. J Obstet Gynaecol Res. 2016;42(5):573-578. doi: 10.1111/jog.12937 [DOI] [PubMed] [Google Scholar]
- 162.Seckin B, Cicek MN, Dikmen AU, Bostancı EI, Muftuoglu KH. Diagnostic value of sonography for detecting endometrial pathologies in postmenopausal women with and without bleeding. J Clin Ultrasound. 2016;44(6):339-346. doi: 10.1002/jcu.22329 [DOI] [PubMed] [Google Scholar]
- 163.Trabert B, Wentzensen N, Yang HP, et al. . Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk? Int J Cancer. 2013;132(2):417-426. doi: 10.1002/ijc.27623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lindenfeld EA, Langer RD. Bleeding patterns of the hormone replacement therapies in the postmenopausal estrogen and progestin interventions trial. Obstet Gynecol. 2002;100(5, pt 1):853-863. [DOI] [PubMed] [Google Scholar]
- 165.Hickey M, Ameratunga D, Marino JL. Unscheduled bleeding in continuous combined hormone therapy users. Maturitas. 2011;70(4):400-403. doi: 10.1016/j.maturitas.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 166.van Hanegem N, Prins MM, Bongers MY, et al. . The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155. doi: 10.1016/j.ejogrb.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 167.Timmermans A, Opmeer BC, Khan KS, et al. . Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2010;116(1):160-167. doi: 10.1097/AOG.0b013e3181e3e7e8 [DOI] [PubMed] [Google Scholar]
- 168.Lee SC, Kaunitz AM, Sanchez-Ramos L, Rhatigan RM. The oncogenic potential of endometrial polyps: a systematic review and meta-analysis. Obstet Gynecol. 2010;116(5):1197-1205. doi: 10.1097/AOG.0b013e3181f74864 [DOI] [PubMed] [Google Scholar]
- 169.Perri T, Rahimi K, Ramanakumar AV, et al. . Are endometrial polyps true cancer precursors? Am J Obstet Gynecol. 2010;203(3):232.e1-232.e6. doi: 10.1016/j.ajog.2010.03.036 [DOI] [PubMed] [Google Scholar]
- 170.Kinde I, Bettegowda C, Wang Y, et al. . Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5(167):167ra4. doi: 10.1126/scitranslmed.3004952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fiegl H, Gattringer C, Widschwendter A, et al. . Methylated DNA collected by tampons: a new tool to detect endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(5):882-888. [PubMed] [Google Scholar]
- 172.Arbyn M, Xu L, Verdoodt F, et al. . Genotyping for human papillomavirus types 16 and 18 in women with minor cervical lesions: a systematic review and meta-analysis. Ann Intern Med. 2017;166(2):118-127. doi: 10.7326/M15-2735 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Diagram
eFigure 2. Prevalence of PMB in Women With Stage I vs Stages II-IV Endometrial Cancers
eFigure 3. Prevalence of PMB in Women With Endometrial Cancer, Stratified by Geographic Region
eFigure 4. Prevalence of PMB in Women With Endometrial Cancer by Study Enrollment Period
eFigure 5. Risk of Endometrial Cancer in Women With PMB by Geographic Region
eFigure 6. Risk of Endometrial Cancer in Women With PMB, Stratified by Study Enrollment Period
eFigure 7. Risk of Endometrial Cancer in Women With PMB by Potential for Study Verification Bias
eFigure 8. Risk of Endometrial Cancer in Women With Postmenopausal Bleeding (PMB) by Hormone Therapy Selection Criteria
eMethods. Study Retrieval and Evaluation and Data Analysis
eTable 1. Results of Quality Assessment of the 92 Studies Included in the Analysis of Risk of Endometrial Cancer in Women With PMB
eTable 2. Results of Sensitivity Analyses Based on Quality Assessment