This case report describes a woman with multiple, inoperable cutaneous basaloid squamous cell carcinomas who was treated with 9-valent human papillomavirus vaccine.
Key Points
Question
Does the 9-valent human papillomavirus vaccine have therapeutic utility for cutaneous squamous cell carcinoma?
Findings
A woman in her 90s with multiple cutaneous squamous cell carcinomas was treated with the 9-valent human papillomavirus vaccine systemically and intratumorally. This approach led to regression of all tumors.
Meaning
These findings suggest that the 9-valent human papillomavirus vaccine could be a therapeutic option for patients with cutaneous squamous cell carcinoma when surgical management is not an option.
Abstract
Importance
Squamous cell carcinoma (SCC) is the second most common form of skin cancer, and its incidence is increasing. When surgical management is not an option, finding a safe and efficacious treatment is a challenge. Mounting evidence suggests that the human papillomavirus (HPV) is involved in the pathogenesis of some SCCs.
Objective
To assess whether the 9-valent HPV vaccine could be an effective treatment strategy for cutaneous SCC.
Design, Setting, and Participants
A woman in her 90s with multiple, inoperable cutaneous basaloid SCCs was successfully treated at a university-based outpatient dermatology clinic with a combination of systemic and intratumoral delivery of the 9-valent HPV vaccine from March 17, 2016, through February 27, 2017, and then followed up through May 21, 2018.
Main Outcomes and Measures
Reduction in tumor size and number after a combination of systemic and intratumoral administration of the HPV vaccine.
Results
All tumors resolved 11 months after the first intratumoral injection of the vaccine. The patient remained free of tumors at the end of follow-up.
Conclusions and Relevance
This is the first report, to our knowledge, of complete regression of a cutaneous malignant tumor after combined systemic and direct intratumoral injection of the 9-valent HPV vaccine. This report suggests that the HPV vaccine may have therapeutic utility for SCCs in patients who are poor surgical candidates, have multiple lesions, or defer surgery.
Introduction
Although surgery remains the standard of care for squamous cell carcinoma (SCC) and basal cell carcinoma (BCC), for patients who are poor surgical candidates, have multiple lesions, or defer surgery, alternative treatments are limited. Recent evidence suggests that the human papillomavirus (HPV) is involved in the pathogenesis of SCC.1,2,3 The 9-valent HPV vaccine is approved by the US Food and Drug Administration to prevent genital warts and anogenital cancer caused by HPV infection. We previously reported that receiving the intramuscular HPV vaccine reduced the development of new SCCs in immunocompetent patients without known HPV infection.4 These cases and the fact that other types of vaccines delivered directly into tumors elicit immune responses capable of eradicating tumor cells led to our using a combination of systemic and intratumoral HPV vaccine for a patient with inoperable SCCs.
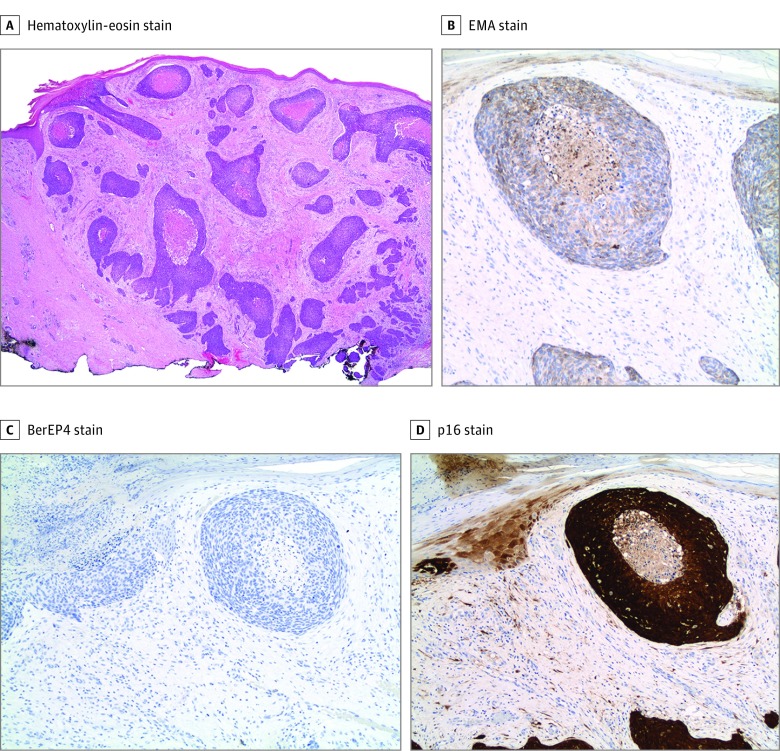
An immunocompetent woman in her 90s presented to a university-based outpatient dermatology clinic with numerous large, nontender, exophytic tumors on her right leg (Figure 1A). Histopathologic examination demonstrated tumor islands of basaloid cells with central comedolike necrosis within a fibrotic dermis, extending from the undersurface of the epidermis (Figure 2A and Figure 3A). Immunohistochemical analysis results were positive for epithelial membrane antigen, negative for Ber-EP4, and strongly positive for p16 (Figure 2B-D), confirming the diagnosis of basaloid SCC, an aggressive SCC with a high recurrence rate after Mohs micrographic surgery and a propensity to metastasize.5 Positron emission tomography–computed tomography findings showed no abnormalities, eliminating the possibility that these tumors represented metastatic basaloid SCC from a distant, noncutaneous site. Mohs surgery was performed on the largest tumor, but considering the patient’s advanced age and extensive tumor burden, additional surgery and radiotherapy were considered to be unfeasible. After the patient declined systemic chemotherapy, she was treated off-label with the 9-valent HPV vaccine delivered systemically and then intratumorally to the largest tumors.
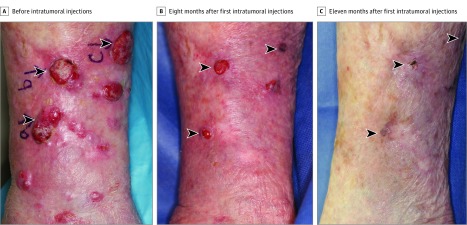
Figure 1. Complete Regression of Multiple Basaloid Squamous Cell Carcinoma Tumors After Combined Systemic and Intratumoral Delivery of the 9-Valent Human Papillomavirus Vaccine.
Arrowheads indicate tumors that were directly injected with the vaccine. Complete tumor regression was observed 11 months after the first intratumoral dose of vaccine.
Figure 2. Histopathologic and Immunohistochemical Analyses of Basaloid Squamous Cell Carcinoma Tumors at Baseline.
A, Infiltrating islands of basaloid cells in a fibrotic stroma extending to the deep dermis (hematoxylin-eosin, original magnification ×20). B, Positive epithelial membrane antigen (EMA) stain findings. C, Negative BerEP4 stain findings. D, Positive p16 stain findings (original magnification ×200).
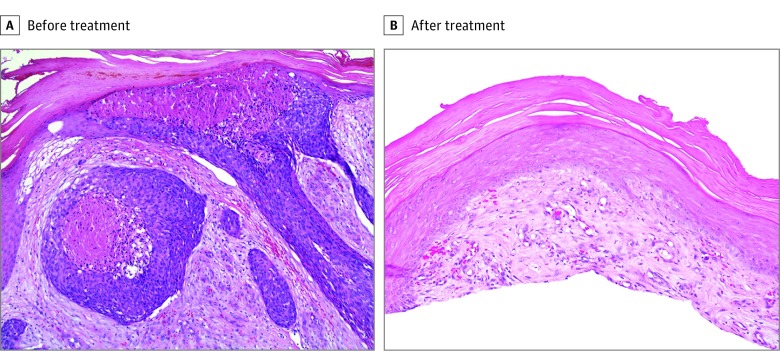
Figure 3. Histopathologic Analysis Before and After Combined Systemic and Intratumoral Administration of the 9-Valent Human Papillomavirus Vaccine.
A, High-power histologic image of a tumor before treatment shows central comedolike necrosis and atypical basaloid cells with mitoses. The tumor arises from the epidermis (hematoxylin-eosin, original magnification ×200). B, High-power histologic image of a previously injected tumor 11 months after the first intratumoral dose of vaccine shows no residual squamous cell carcinoma but mild cellular atypia of basal keratinocytes and hyperkeratosis (hematoxylin-eosin, original magnification ×200).
Methods
With the oral informed consent of the patient and her son, the patient was treated with the 9-valent HPV vaccine (Gardasil-9; Merck & Co Inc) from March 17, 2016, through February 27, 2017, and was followed up through May 21, 2018. She was initially treated with 2 doses of intramuscular HPV vaccine administered 6 weeks apart. Three weeks after the second intramuscular dose, 3 of the largest tumors were treated intratumorally with 9-valent HPV vaccine (0.5 mL) diluted with sterile saline (2.5 mL) (Figure 1A) after the tumors had been anesthetized with lidocaine, 1%, with epinephrine (1:100 000). Three additional similar doses of the HPV vaccine were administered intratumorally during the ensuing 8 months (Figure 1B), leading to complete resolution (Figure 1C). No additional treatments were used.
Results
Clinical improvement was observed 2 weeks after the second intratumoral dose of the 9-valent HPV vaccine with reduction in tumor size and number. Eleven months after the first intratumoral dose, there was no clinical or histologic evidence of residual SCC. On examination, there were small violaceous scars and 1 small, pink, scaly, minimally elevated papule on her right leg at sites corresponding to previous large tumors (Figure 1A-C). Histopathologic analysis of the pink, scaly papule was performed, the results of which demonstrated mild cellular atypia of basal keratinocytes with hyperkeratosis (Figure 3). The only adverse effect was mild pain within some of the tumors on the day of intratumoral vaccine administration. No systemic adverse effects were reported. At the patient’s most recent follow-up visit, 24 months after the first intratumoral administration of the HPV vaccine, there was no clinical evidence of SCC recurrence.
Discussion
We report the first case, to our knowledge, of complete regression of multiple cutaneous SCCs after combined systemic and intratumoral delivery of the 9-valent HPV vaccine. The marked regression of numerous SCCs after initiation of the intratumoral injections eliminated the need for additional treatment. Tumors not directly injected with the vaccine also regressed, possibly by local dispersion of the vaccine or its effects on immune-mediated mechanisms. These findings suggest that the 9-valent HPV vaccine can provide a therapeutic option for inoperable cutaneous SCCs, in addition to its approved use to prevent anogenital HPV infection.
The development of therapeutic vaccines has created a new era for cancer treatments. In 2015, the US Food and Drug Administration approved the first oncolytic virus vaccine for the treatment of inoperable metastatic melanoma, talimogene laherparepvec, an engineered herpes virus injected directly into tumors.6 An attenuated polio-rhinovirus oncolytic vaccine is injected directly into glioblastoma multiforme.7 Although associated with an unfavorable adverse effect profile, the BCG vaccine has been used intratumorally for metastatic melanoma and intravesically for bladder carcinoma.8
Human papillomavirus is a highly prevalent virus that colonizes skin and mucosa. More than 200 HPV types have been identified, including high-risk carcinogenic types with a well-established role in the development of anogenital and oropharyngeal carcinomas. It is estimated that approximately 5% of human malignant tumors may be associated with HPV.9 Numerous studies1,3,10,11 have described the association between the β-genus of HPV (β-HPV) and the development of keratinocyte carcinomas. The β-HPV types 5, 8, 15, 17, 20, 24, 36, and 38 are associated with an increased risk of developing cutaneous SCC,2 and the risk is higher in individuals who are seropositive for multiple β-HPV subtypes.10 The β-HPV DNA, mainly subtypes 5 and 8, can be found in 90% of keratinocyte carcinomas in immunocompromised patients and 50% of keratinocyte carcinomas in immunocompetent patients.11
Several mechanisms have been proposed to explain the role of HPV in the development of keratinocyte carcinomas, including the expression of oncoproteins E6 and E7, which can deregulate gene expression, stimulate keratinocyte proliferation, and immortalize keratinocytes, creating a favorable environment for viral replication, particularly when UV damage is present.2,12 The 9-valent HPV vaccine treats a variety of mucocutaneous conditions, including recalcitrant cutaneous warts and oral papillomas. We found that vaccination against HPV can also prevent the development of SCCs and BCCs in immunocompetent individuals.4
Conclusions
It is not known what part, if any, the systemic doses of the vaccine played in the therapeutic benefit that we observed after the intratumoral injections in the patient. The prophylactic role of HPV vaccination is well understood; however, the mechanisms of its therapeutic efficacy in cutaneous malignant tumors are not yet clear. The potent therapeutic benefit may reflect a combination of immunologic, antiviral, and antitumor effects of 9-valent HPV vaccine.
References
- 1.Borgogna C, Lanfredini S, Peretti A, et al. . Improved detection reveals active β-papillomavirus infection in skin lesions from kidney transplant recipients. Mod Pathol. 2014;27(8):1101-1115. doi: 10.1038/modpathol.2013.240 [DOI] [PubMed] [Google Scholar]
- 2.Chahoud J, Semaan A, Chen Y, et al. . Association between β-genus human papillomavirus and cutaneous squamous cell carcinoma in immunocompetent individuals: a meta-analysis. JAMA Dermatol. 2016;152(12):1354-1364. doi: 10.1001/jamadermatol.2015.4530 [DOI] [PubMed] [Google Scholar]
- 3.Harwood CA, Surentheran T, McGregor JM, et al. . Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61(3):289-297. doi: [DOI] [PubMed] [Google Scholar]
- 4.Nichols AJ, Allen AH, Shareef S, Badiavas EV, Kirsner RS, Ioannides T. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153(6):571-574. doi: 10.1001/jamadermatol.2016.5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand RL, Collins D, Chapman A. Basosquamous carcinoma: appearance and reality. Oxf Med Case Reports. 2017;2017(1):omw095. doi: 10.1093/omcr/omw095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841-849. doi: 10.1001/jamaoncol.2016.2064 [DOI] [PubMed] [Google Scholar]
- 7.Brown MC, Gromeier M. Cytotoxic and immunogenic mechanisms of recombinant oncolytic poliovirus. Curr Opin Virol. 2015;13:81-85. doi: 10.1016/j.coviro.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer: a current perspective. Nat Rev Urol. 2014;11(3):153-162. doi: 10.1038/nrurol.2014.15 [DOI] [PubMed] [Google Scholar]
- 9.Drvar DL, Lipozenčić J, Sabol I, Mokos ZB, Ilic I, Grce M. Human papillomavirus status in extragenital nonmelanoma skin cancers. Clin Dermatol. 2014;32(2):248-252. doi: 10.1016/j.clindermatol.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 10.Karagas MR, Waterboer T, Li Z, et al. ; New Hampshire Skin Cancer Study Group . Genus beta human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: population based case-control study. BMJ. 2010;341:c2986. doi: 10.1136/bmj.c2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karagas MR, Nelson HH, Sehr P, et al. . Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98(6):389-395. doi: 10.1093/jnci/djj092 [DOI] [PubMed] [Google Scholar]
- 12.Cornet I, Bouvard V, Campo MS, et al. . Comparative analysis of transforming properties of E6 and E7 from different beta human papillomavirus types. J Virol. 2012;86(4):2366-2370. doi: 10.1128/JVI.06579-11 [DOI] [PMC free article] [PubMed] [Google Scholar]