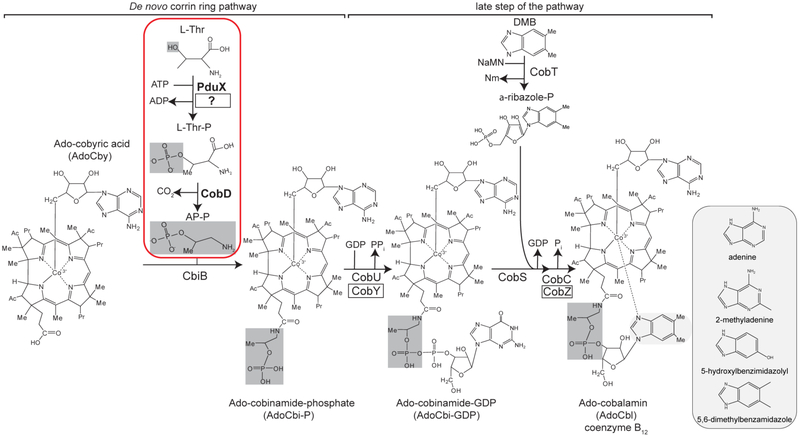
Figure 1.
Assembly of the nucleotide loop in bacteria and archaea - Non-homologous archaeal enzyme names are boxed in black. The relevant reactions are boxed in red with enzyme names in bold. Highlighted in dark gray are the hydroxyl group of L-threonine (L-Thr) that is phosphorylated by PduX in S. enterica and the MmCobD archaeal kinase, the resulting phosphate of L-threonine-O-3-phosphate (L-Thr-P), and the (R)-1-aminopropan-2-ol O-phosphate (AP-P) linker which is subsequently attached to the corrinoid ring to form the linker between the ring and nucleotide base. The purine analog base 5,6-dimethylbenzimidazole that is particular to cobalamin (Cbl) is highlighted in a light gray oval. Inset: Boxed in light gray are purine and purine analog bases incorporated in cobamides synthesized by S. enterica and M. mazei. AdoCby, adenosylcobyric acid; AdoCbi-P, adenosylcobinamide phosphate; AdoCbi-GDP, adenosylcobinamide-GDP; AP-P, (R)-1-aminopropan-2-ol O-phosphate; L-Thr-P, L-threonine-O-3-phosphate; L-Thr, L-threonine; α-ribazole phosphate, DMB, 5,6-dimethylbenzimidazole; NaMN, nicotinic acid mononucleotide; Nm, Nicotinic Acid; PPi, pyrophosphate; Pi, orthophosphate; CbiB, AdoCbi-P synthase; CobY, AdoCbi-P guanylyltransferase; CobS, AdoCba-5’P synthase; CobD, L-Thr-P decarboxylase; CobT, NaMN:DMB phosphoribosyltransferase; CobU, AdoCbi kinase / AdoCbi-P guanylyltransferase; PduX, L-Thr kinase; CobC, AdoCba-5’-P phosphatase; CobZ; AdoCba-5’-P phosphatase.