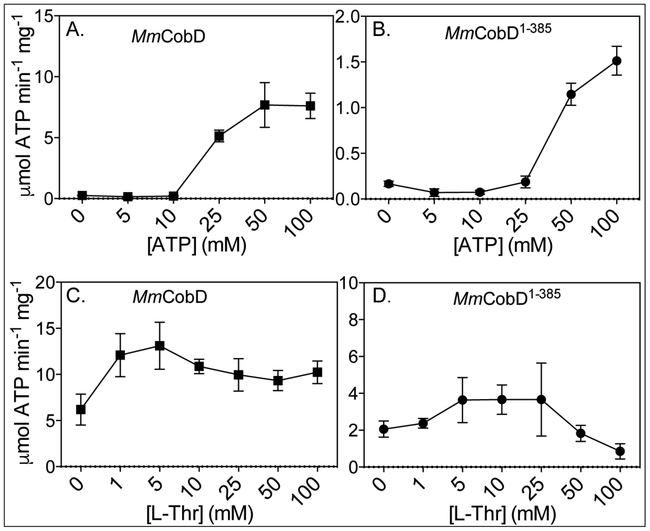
Figure 9.
Specific activities of normoxically purified MmCobD and MmCobD1-385 proteins as a function of substrate concentrations - Indirect measurement of the specific activity of (A) MmCobD and (B) MmCobD1-385 proteins as a function of ATP concentration, and (C), (D) L-Thr concentration expressed as μmol of ATP per min per mg of protein with the standard deviation from the mean (SD) of triplicate reactions represented by the error bars. Activity was measured by a NADH-consuming assay described in the Materials and Methods section. Assays were performed with normoxically purified protein (3 μM), HEPES buffer (50 mM pH 7.5 at 25°C), MgCl2 (5 mM), phosphoenolpyruvate (PEP, 3 mM), NADH (0.1 mM), pyruvate kinase (1 U), lactate dehydrogenase (1.5 U) incubated at 25°C for 20 min under normoxic conditions. For ATPase specific activity L-Thr concentration was held at 50 mM while ATP concentration was varied (5-100 mM). To measure the effect of the co-substrate on ATPase activity, ATP concentration was held at 50 mM while the concentration of L-Thr was varied (0-100 mM).