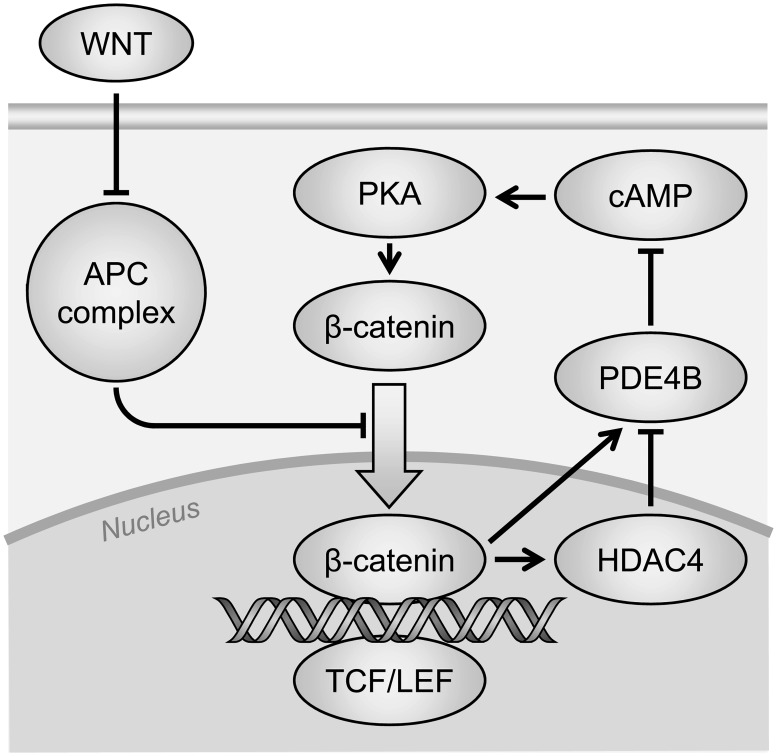
Fig 11. Feedback model for the interaction of Wnt and cyclic AMP signaling.
The APC complex mediates the destruction of β-catenin. Its activity in turn is neutralized by WNT signaling allowing for β-catenin accumulation and nuclear translocation. This double-negative chain makes WNT a positive-regulator of β-catenin activity. When APC is truncated, increased β-catenin-mediated transcription ensues, causing tumorigenesis in the colon. Promoter-binding assays [51] are interpreted to show that nuclear β-catenin activates the transcription of PDE4B, whose hydrolytic activity inactivates cyclic AMP (cAMP). Since cAMP is a positive effector of protein kinase A, which in turn activates β-catenin, the hydrolysis of cAMP blunts the activation of β-catenin. The observed silencing of PDE4B in advanced colonic cancer, which would be predicted to enhance β-catenin activity, may involve the repressive histone deacetylase HDAC4, that is also β-catenin target gene.