Table 3.
Optimization of Photoinduced Method To Produce 2-Phenyl-1,2-benzisoselenazol-3(2H)-onesa
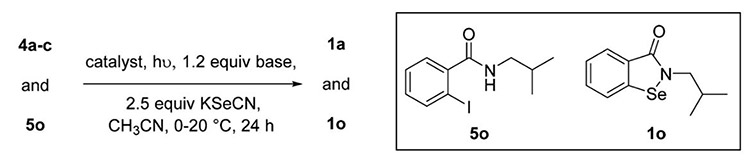 |
||||
|---|---|---|---|---|
| entry | equiv, catalyst | halogen | energy | yield (%) |
| 1 | 1.0, phen-CuI | 4b | thermal reaction | 1a: 89b |
| 2 | 1.0, phen-CuI | 4b | 22 W Hg lamp | 1a: 31 |
| 3 | 1.0, CuI | 4b | 22 W Hg lamp | 1a: 82 |
| 4 | 0.1, CuI | 4b | 22 W Hg lamp | 1a: 60c |
| 5 | 4b | 22 W Hg lamp | 1a: N.R. | |
| 6 | 1.0, CuI | 4b | dark | 1a:6 |
| 7 | 1.0, CuI | 4b | 22 W Hg lamp | 1a: N.R. |
| 8 | 1.0, CuI | 4b | ambient light | 1a: 21 |
| 9 | 1.0, CuI | 4b | 250 W infrared lamp | 1a: 63d |
| 10 | 0.1, CuI | 4b | 22 W Hg lamp | 1a: 81c,e |
| 11 | 1.0, CuI | 4c | 22 W Hg lamp | 1a: 92 |
| 12 | 1.0, CuI | 4a | 22 W Hg lamp | 1a: N.R. |
| 13 | 1.0, CuI | 5o | 22 W Hg lamp | 1o: 85 |
| 14 | 1.0, CuI | 4b | 14 W Hg lamp | 1a: 77f |
Unless otherwise stated, KSeCN (2.5 equiv) and Cs2CO3 (1.2 equiv) 24 h, and acetonitrile were used. Reactions were cooled for about 2 h and allowed to warm to room temperature (20 °C).
Thermal activation = phen-CuI (1.0 equiv), KSeCN (1.2 equiv), Cs2CO3 (2.5 equiv), 12 h, 100–110 °C.
48 h.
Reaction reached 65–70 °C.
NaOtBu (0.1 equiv). N.R = no reaction. 22 W is a combined wattage of two lamps.
Reaction performed without BLE-8T365 (320–400 nm) lamp; only the 14 W Rayonet RPR-3000A lamp was used.
