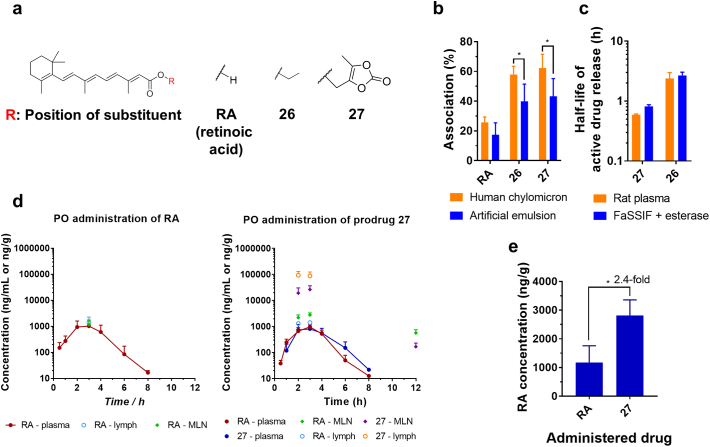
Fig. 8.
Application of the prodrug approach to RA (retinoic acid). (a) Chemical structures of RA and its prodrugs. (b) Association with human chylomicrons and artificial emulsion (mean ± SD, n = 6). (c) Half-life of active drug release in rat plasma and fasted state simulated intestinal fluid (FaSSIF) with esterase (mean ± SD, n = 3). (d) In vivo pharmacokinetic and biodistribution profiles in plasma and intestinal lymphatics following oral administration of RA (10 mg/kg) or prodrug 27 (at equivalent to 10 mg/kg RA) in rats (mean ± SD, n = 5 for plasma and n = 4 for mesenteric lymph nodes (MLN) and lymph). (e) Maximum concentration of RA observed in MLN (ng/g) during biodistribution studies following oral administration of RA and prodrug 27 (mean ± SD, n = 4). *, p < .05.