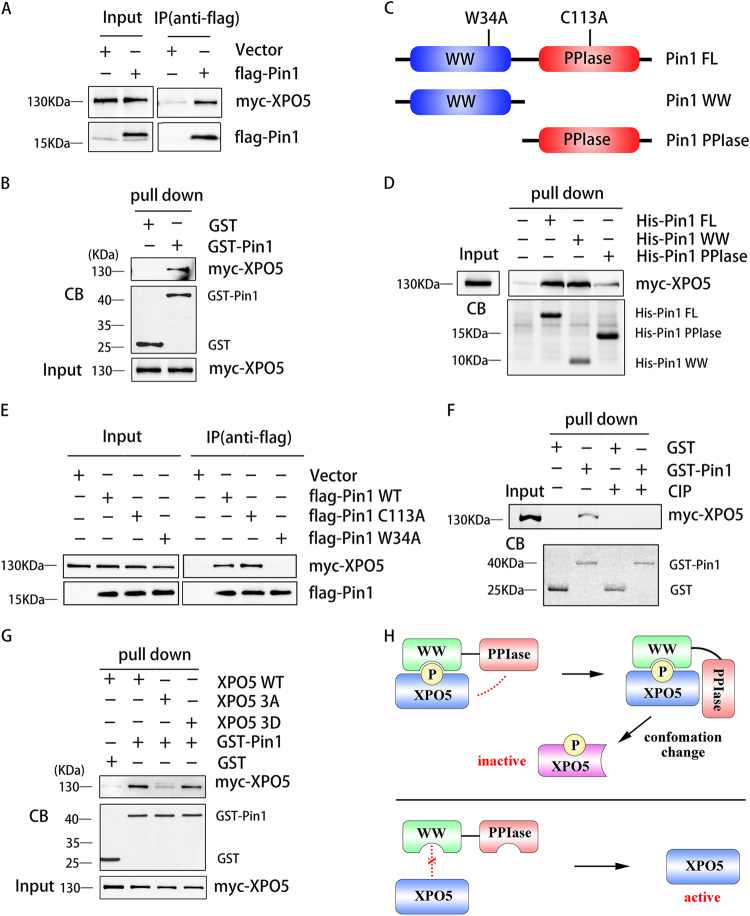
Fig. 2.
Interaction between Pin1 and XPO5 occurs in a phosphorylation-dependent manner. a SK-Hep1 cells were co-transfected with myc-XPO5 and flag-Pin1 or empty vector expression plasmid. After cell lysis, flag-Pin1 was immunoprecipitated with anti-flag antibody. Immunoprecipitates were immunoblotted with anti-myc and anti-flag antibodies. b Lysates from SK-Hep1 cells transfected with myc-XPO5 expression plasmid were incubated with GST or GST-Pin1. GST pull-down complexes were subjected to SDS-PAGE and immunoblotted with anti-myc antibody or stained by Coomassie blue (CB). c Schematic diagram of full length Pin1 and its mutants (W34A and C113A), Pin1 WW domain, and Pin1 PPIase domain. d Lysates from SK-Hep1 cells transfected with myc-XPO5 expression plasmid were incubated with His-tagged full-length Pin1 (His-Pin1 FL), WW domain (His-Pin1 WW), or PPIase domain (His-Pin1 PPIase), respectively. The pull-down complexes by Ni-NTA agarose beads were subjected to SDS-PAGE and immunoblotted with anti-myc antibody or stained by Coomassie blue (CB). e SK-Hep1 cells were co-transfected with myc-XPO5 and flag-Pin1 (WT, C113A, or W34A) or empty vector expression plasmid. After cell lysis, flag-Pin1 was immunoprecipitated with anti-flag antibody. Immunoprecipitates were immunoblotted with anti-myc and anti-flag antibodies. f Lysates from SK-Hep1 cells transfected with myc-XPO5 expression plasmid were incubated with or without CIP before the incubation with GST or GST-Pin1. GST pull-down complexes were subjected to SDS-PAGE and immunoblotted with anti-myc antibody or stained by Coomassie blue (CB). g Lysates from SK-Hep1 cells transfected with myc-XPO5 (WT, 3A, or 3D) or empty vector expression plasmid were incubated with GST or GST-Pin1. GST pull-down complexes were subjected to SDS-PAGE and immunoblotted with anti-myc antibody or stained by Coomassie blue (CB). 3A: T345A, S416A and S497A mutations; 3D: T345D, S416D and S497D mutations; h Graphic abstract of the mechanism how Pin1 interacts with XPO5