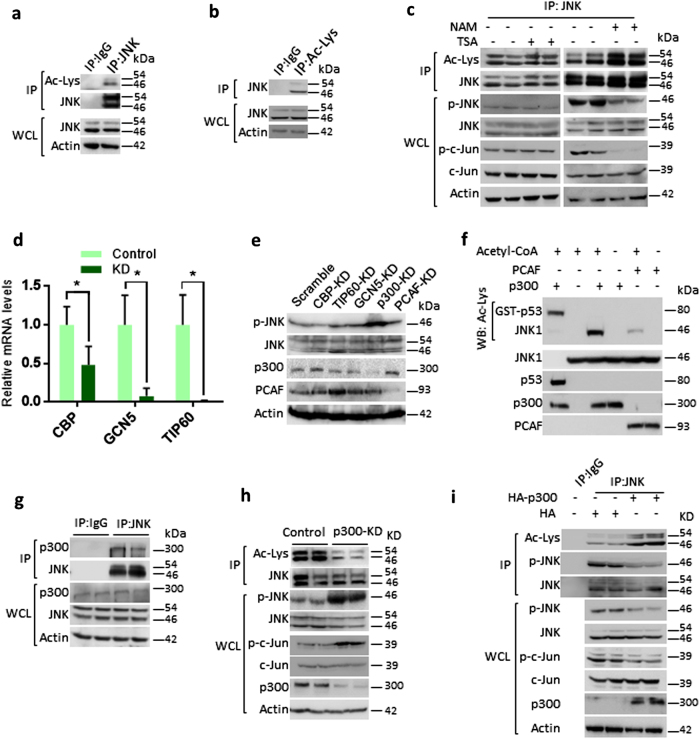
Fig. 1.
p300 acetyltransferase play key role in regulating JNK acetylation and activity. a Western blotting analysis showing the acetylation of endogenous JNK in HEK 293T cells. Endogenous JNK was immunoprecipitated and acetylation was detected by western blotting using pan-acetyl lysine antibody. Whole-cell lysates (WCLs) of 293T cells were probed for JNK and actin. n = 3 independent experiments. b Western blotting analysis depicting the acetylation status of JNK in 293T cells. Endogenous acetylated proteins were immunoprecipitated with anti-pan acetyl lysine antibody and probed for JNK by western blotting. WCLs of 293T cells were probed for JNK and actin. n = 3 independent experiments. c Western blot analysis showing the acetylation, phosphorylation status and activity of JNK in cells treated with Class I and II HDAC inhibitor, Trichostatin A (TSA) or Class III HDAC inhibitor, nicotinamide (NAM). 293T cells were treated with vehicle or 10 µM TSA for 12 h. 293T cells were treated with vehicle or 50 mM NAM for 6 h. Acetylation was assessed by immunoprecipitating endogenous JNK with a specific JNK antibody. The WCL was tested for the phosphorylation of JNK by western blotting. The activity of endogenous JNK was tested by measuring the phosphorylation of c-Jun, a downstream transcription factor of JNK. n = 4 independent experiments. d Graph showing the relative mRNA expression levels of acetyl transferases, CBP, GCN5 and TIP60 in HeLa cells treated with either scramble (control) or specific pool of shRNAs targeting individual acetyl transferases, as measured by real-time qPCR analysis. n = 3–4 samples per group. Data are presented as mean ± s.d. *p < 0.05. Student’s t-test was used to calculate the p-values. e Western blotting analysis showing the phosphorylation status and activity of JNK in HeLa cells treated with scramble or specific pool of shRNAs targeting individual acetyl transferases. The WCL was used to check the phosphorylation of JNK by western blotting. Depletion of p300 or PCAF was tested by assessing their protein levels by western blotting with specific antibodies. The depletion of acetyl transferases, CBP, GCN5 and Tip60 was assessed by measuring the mRNA levels, as shown in Fig. 1d, due to lack of specific antibodies. f Western blotting analysis showing in vitro acetylation of JNK by p300. Purified recombinant JNK was used as a substrate for acetyltransferases p300 and PCAF in the presence or absence of acetyl CoA. The acetylation of JNK was assessed by western blotting with pan-acetyl lysine antibody. Recombinant p53 was used as a positive control for acetylation assay. Recombinant p300 strongly acetylates JNK, when compared to PCAF, as assessed by western blotting. g Endogenous JNK was immunoprecipitated and probed for its interaction with p300 by western blotting. IgG was used as negative control. WCLs were probed for the presence of JNK and p300 by western blotting. h Western blotting analysis showing acetylation, phosphorylation and activity of JNK in HeLa cells expressing luciferase shRNA (control) or p300 shRNA (p300-KD). WCLs were probed for the depletion of p300 by western blotting. JNK was immunoprecipitated and probed for Ac-Lys antibody. JNK activity was measured by assessing the phosphorylation of c-Jun. Specific antibody was used to detect the phosphorylation of JNK. i Western blotting analysis showing acetylation and phosphorylation of JNK in HeLa cells overexpressing HA-p300. JNK was immunoprecipitated from HeLa cells transiently overexpressing p300 were analysed for the acetylation and phosphorylation levels of JNK by western blotting. WCLs were probed for the overexpression of p300