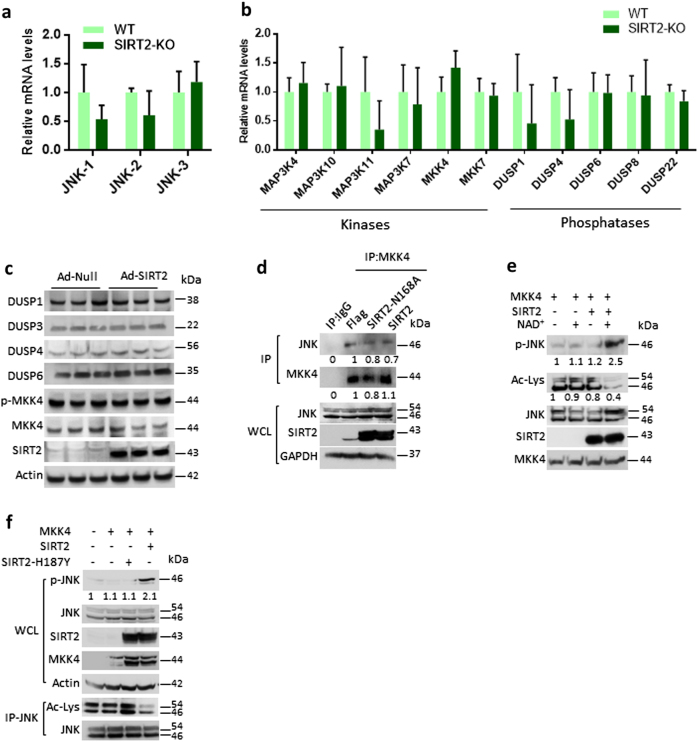
Fig. 3.
Deacetylation favours MKK4-dependent activation of JNK. a Graph showing the relative mRNA expression levels of JNK isoforms in wild-type (WT) and SIRT2-deficient (SIRT2-KO) mice, as measured by real-time qPCR analysis. n = 3–4 mice per group. Data are presented as mean ± s.d. *p < 0.05. Student’s t-test was used to calculate the p-values. b Graph showing the relative mRNA expression levels of JNK upstream kinases and phosphates in WT and SIRT2-KO mice, as measured by real-time qPCR analysis. n = 3–4 mice per group. Data are presented as mean ± s.d. *p < 0.05. Student’s t-test was used to calculate the p-values. c Western blotting images showing the expression of JNK upstream phosphates in HeLa cells overexpressing either control (Ad-Null) or SIRT2 (Ad-SIRT2). Similarly, the phosphorylation of MKK4, an upstream kinase of JNK, was measured by western blotting. n = 3 independent experiments. d Representative western blotting images showing MKK4 interaction with JNK. MKK4 was immunoprecipitated from cells transiently expressing SIRT2 or SIRT2-N168A mutant and probed for JNK by western blotting. Whole-cell lysates (WCLs) were probed for the overexpression of SIRT2 and endogenous JNK levels by western blotting. n = 3 independent experiments. e Representative western blotting images showing the effect of acetylation on the MKK4-mediated phosphorylation of JNK. Endogenous acetylated JNK was immunoprecipitated from HeLa cells treated with deacetylase inhibitors. The acetyl-JNK was further incubated with SIRT2 in the presence or absence of NAD+ in a test tube and then subjected to a kinase assay, where deacetylated JNK was used as substrate for MKK4. Phosphorylation and acetylation of JNK were assessed using western blotting. n = 3 independent experiments. f Representative western blotting images showing the effect of SIRT2-mediated deacetylation on the MKK4-dependent phosphorylation of JNK. MKK4 was overexpressed in HeLa cells transiently expressing either SIRT2 or SIRT2-H187Y. WCLs were probed for the overexpression of SIRT2, SIRT2-H187Y and MKK4. Endogenous JNK levels and its phosphorylation was assessed by western blotting. Lower panel shows the acetylation status of JNK assessed by western blotting