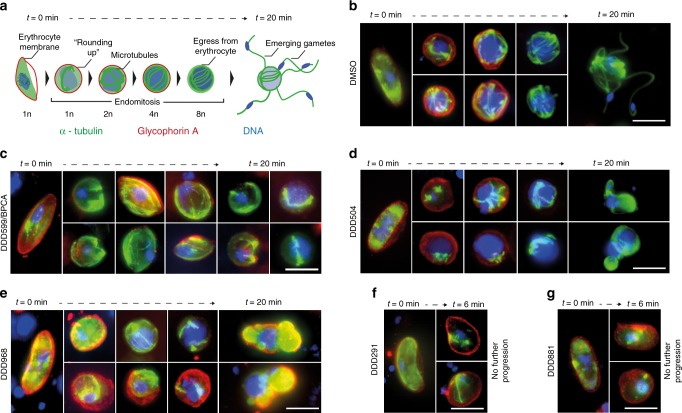
Fig. 4.
Monitoring the effect of compound treatment on male gamete formation by IFA. Gametocytes were treated for 24 h with DMSO or 10 µM test compound and then gamete formation triggered by the addition of ookinete medium and reduction to room temperature. Aliquots were sampled at different time points and immediately fixed before being stained directly for α-tubulin (green), glycophorin A (red) and DNA via DAPI labelling (blue). Images show representative non-induced cells (0 min), representative cells during gamete formation progression (2–10 min), and representative cells showing the most advanced phenotype at 20 min. a Upon activation, male gametocytes initially round up before undergoing three endomitotic genome replications, egressing from the surrounding erythrocyte membrane and releasing up to eight flagellated gametes. b Images from left to right show DMSO-treated cells undergoing normal male gamete formation over 20 min. Male gametocytes initially have an elongated morphology with a faint nucleus, relatively homogenous tubulin distribution and are surrounded by an erythrocyte membrane. Upon induction of gametogenesis, cells round up and begin three rounds of endomitosis, with ordered microtubule spindle fibres and increasingly intense DNA staining. Towards the end of gametogenesis, the surrounding erythrocyte membrane is shed and up to eight microgametes emerge from the cell possessing a microtubule-rich flagellum and one replicated genome. c DDD599/BPCA-treated cells; d DDD504-treated cells; e DDD968-treated cells; f DDD291-treated cells; g DDD881-treated cells. Bars = 5 µm