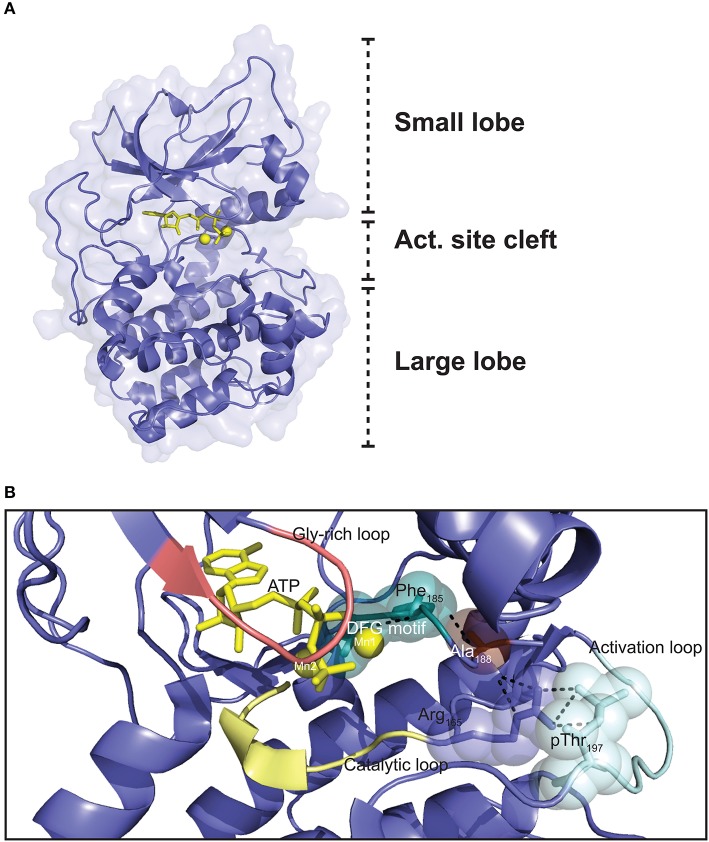
Figure 3.
Three dimensional structure of the PKA C subunit. (A) The C subunit is composed of a small lobe, large lobe, and an active site cleft with a binding site for an ATP molecule (yellow sticks) and two Mg2+ ions (yellow spheres). The figure is based on the experimental structure with Protein Data Bank (PDB) identifier 3FJQ (169). (B) Schematic representation of the active site cleft of PKA Cα1. Motifs and residues described in the text are indicated. Dashed lines indicate the chain of interactions leading from pThr197 to Phe185 in the DFG motif when the enzyme is in the active conformation. The structure is solved with Mn2+ as the divalent cations, although Mg2+ is thought to be the most relevant biological chelating agent (170). ATP and the Mn2+ ions are shown in yellow, and the DFG motif (teal), Gly-rich loop (salmon), catalytic loop (yellow), and activation loop (cyan) are also highlighted. PDB identifier 3FJQ (169).