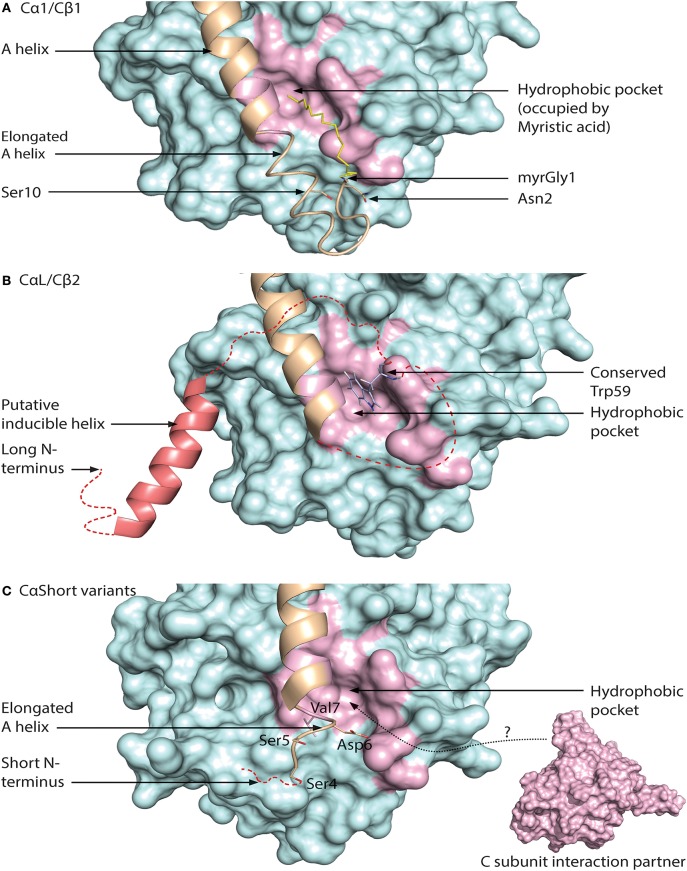
Figure 5.
Different configurations of N terminal parts of C subunit isoforms. For all figures, the C subunit is represented in cyan with the hydrophobic pocket highlighted in purple. Alternative exon 1 encoded parts of the C subunit are in orange cartoon presentation, in addition to the mainly exon 2 encoded A helix. Hypothesized (i.e., not supported by published crystal structure data) structures of N-terminal residues are colored red. (A) Representation of human myristoylated Cα1. The structure of unphosphorylated (Ser10), unmodified (i.e., not deamidated) Asn2, and Gly1-myristoylated Cα1 shows a fully ordered N-terminus. The modifiable residues in the 5′ encoded exon are highlighted in stick presentations, and myristic acid (yellow) occupies the hydrophobic pocket. Based on the structure with PDB identifier 1CMK (73). (B) Proposed model of N-terminal structure of CαL/Cβ2 homologs. Our study identified a conserved Trp59 (human Cβ2 numbering) (stick presentation, slate) residue which potentially occupies the hydrophobic pocket. The most conserved part of the N-terminus was predicted to encode a helix structure, which we hypothesize may be ordered upon binding to interaction partners. The figure is modeled from the experimental structure of Cα1, with the N-terminal residues encoded by exon 1 modeled. PDB identifier 1CMK (73). (C) Proposed model of N-terminal structure of CαShort variants. Short N-terminal transcripts in Cα were identified in most vertebrate species investigated. The short N-terminal end displays the open hydrophobic pocket as earlier proposed for Cα2. The figure is based upon the experimental structure of human Cα2 with PDB identifier 4AE9 (63).