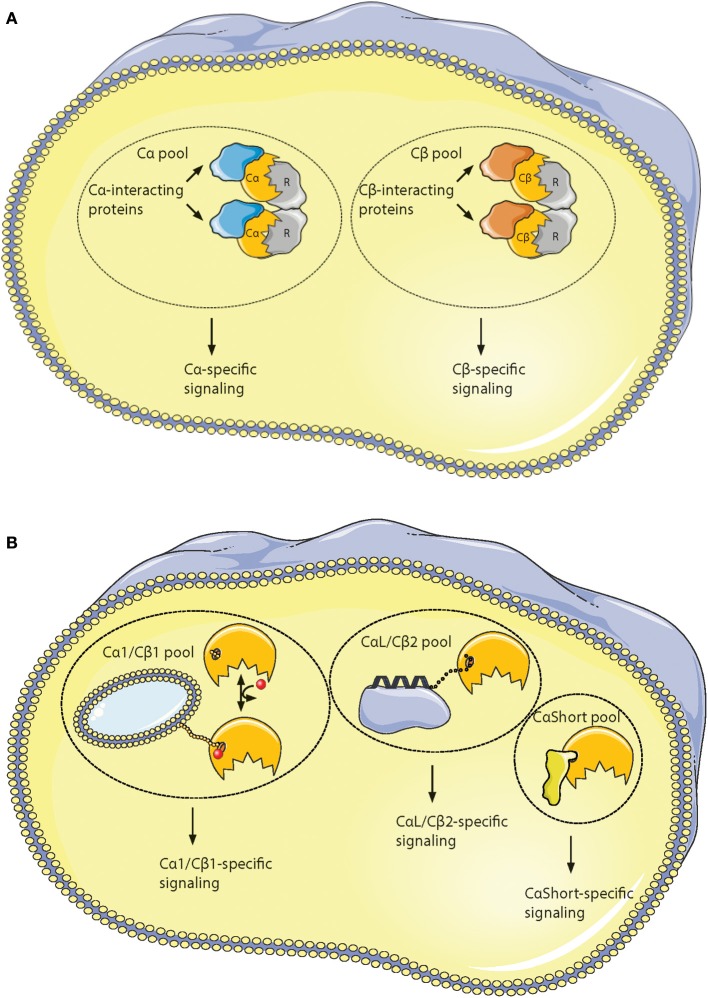
Figure 6.
Hypothesis of localized pools of isoform-specific PKA signaling. (A) Most of the variations in the Core16−350 residues in Cα and Cβ proteins are located to 11 solvent exposed residues in the small lobe. This opens for the possibility of Cα- and Cβ-specific interaction partners interacting with the small lobe [described in (196)], possibly locating the two subunits into separate intracellular signaling pools. (B) Evolution of alternative N-termini in Cα and Cβ provides another mechanism for acquiring localized pools of isoform-specific PKA signaling [described in (188)]. The Cα1 and Cβ1 pool (left) shares the myristic acid with a regulatory mechanism, evolved in mammals, through phosphorylation/dephosphorylation of Ser10 for switching myristic acid in and out of the hydrophobic pocket (phosphate group presented as a red dot, and myristic acid presented as a yellow chain). This represents the main source of PKA C activity in most human cells. The conserved, putative inducible α-helix opens for the possibility of a CαL/Cβ2-specific pool (middle), docking the C subunits via a flexible linker to a CαL/Cβ2-specific subcellular assembly of proteins (purple). The Cα2 protein (CaShort pool, right) has a conserved sperm-specific expression in all mammals, and possibly interacts with Cα2-specific proteins (bright yellow) binding to the hydrophobic pocket. Similar CαShort-specific proteins may exist in other tissues in non-mammals. Figure created using the Servier Medical Art resource (http://www.servier.com/Powerpoint-image-bank).