Abstract
Aim: Ischemia-reperfusion (I-R) produces reactive oxygen species (ROS) that damage cells and favour cytotoxicity and apoptosis in peripheral artery disease (PAD) patients. Since brief episodes of I-R (ischemic conditioning) protect cells against ischemic harms, we evaluated whether a short-course of supervised treadmill training, characterized by repeated episodes of I-R, makes peripheral blood mononuclear cells (PBMCs) from PAD patients with intermittent claudication more resistant to I-R injuries by reducing oxidative stress and by inducing an adaptative response of unfolded protein response (UPR) and nuclear factor-E2-related factor (Nrf2) pathway expression.
Methods: 24 PAD patients underwent 21 sessions of treadmill training and a treadmill test as indicator of acute response to I-R.
Results: Maximal and pain free walking distance improved (p < 0.01), whereas LDH leakage and apoptosis of PBMCs decreased (p < 0.01); plasma malondialdehyde and ROS generation in PBMCs declined, while plasma glutathione augmented (p < 0.01). Moreover we demonstrated an up-regulation of UPR and Nrf2 expression in PBMCs (p < 0.01). To understand whether treadmill training may act as a trigger of ischemic conditioning, we examined the effect of repeated episodes of I-R on adaptative response in PBMCs derived from the patients. We showed an up-regulation of UPR and Nrf2 gene expression (p < 0.01), while oxidative stress and cytotoxicity, after an initial increase, declined (p < 0.01). This positive effect on cytotoxicity was reduced after inhibition of UPR and Nrf2 pathways.
Conclusions: Treadmill training in PAD patients through UPR and Nrf2 up-regulation may trigger hypoxic adaptation similar to conditioning, thus modifying cell survival.
Keywords: Nrf2, UPR, PAD, Oxidative stress
Introduction
Patients affected by peripheral artery disease (PAD) suffer from calf muscle ischemia during walking activity when metabolic demands outdo oxygen supply, and from calf muscle reperfusion during rest, when blood supply augments enough to satisfy calf muscle oxygen demand. Although reperfusion is essential to salvage calf muscles following periods of sustained ischemia caused by PAD, the actual process of reperfusing ischemic calf muscle can itself paradoxically induce injury. In fact, this phenomenon of ischemia-reperfusion (I-R) produces reactive oxygen species (ROS) that damage muscle fibers, impair mitochondrial function, and favor apoptosis1–6).
Nuclear factor-E2-related factor (Nrf2) is an emerging regulator of cellular resistance to oxidants. Nrf2 controls the basal and induced expression of an array of antioxidant response element (ARE)-dependent genes as heme-oxygenase (HO)-1 and glutamate-cysteine ligase catalytic (GCLC) subunit, to regulate the physiological and pathophysiologic outcomes of oxidant exposure7). Interestingly, it has been recently shown that Nrf2 has a protective role in murine models of myocardial I-R injury8, 9).
Stresses that perturb the folding of nascent endoplasmic reticulum (ER) proteins activate the ER stress response which, in turn, triggers the so called unfolded protein response (UPR) that deals with unfolded and misfolded proteins10). Three main ER transmembrane stress sensors initiate the UPR: protein kinase RNA-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1). Activation of PERK decreases protein synthesis and specifically encodes for activating transcription factor 4 (ATF4), which induces the expression of genes involved in aminoacid metabolism, antioxidant response, autophagy, and apoptosis11). After its activation, IRE1 processes the mRNA encoding X-box-binding protein 1 (XBP1) generating a transcription factor that up-regulates a subset of UPR target genes related to folding, quality control and ER-associated degradation (ERAD). Upon the induction of ER stress, ATF6 is processed at the Golgi apparatus with the release of its cytosolic domain, which then translocates to the nucleus where it increases the expression of some ER chaperones, ERAD-related genes, and XBP111). Once the UPR fails to control the level of unfolded and misfolded proteins in the ER, a pro-apoptotic CCAAT/2enhancerbinding protein homologous protein (CHOP)-mediated pathway is initiated11).
I-R is associated with an increased abundance of structurally and functionally defective proteins, which endanger normal cellular function and must therefore be removed12). In this context, it has been shown that the ATF6 branch of the UPR may induce expression of proteins that can reduce I-R injury in hearts of transgenic mice13) and in cardiac myocytes14). Similarly, activation of the other sensors of the UPR has also been reported in different models of I-R15–17).
Ischemic conditioning is an endogenous phenomenon in which one or more brief episodes of nonlethal I-R protect against a sustained lethal episode of I-R18). Extensive research has focused on increasing heart tolerance to I-R injury using conditioning strategies18): brief episodes of coronary I-R preceding (ischemic preconditioning, IP) sustained myocardial I-R reduce infarct size in mice18), as well as in patients with acute myocardial infarction, and in those undergoing cardiac surgery or percutaneous coronary intervention18). Nevertheless, there are pathological conditions, such as transitory ischemic attack and stable angina, in which patients are exposed to several brief episodes of I-R. In this regard, limb ischemia caused by PAD is likely the most important potential contributor to frequent conditioning episodes19). Despite the obvious fact that conditioning and intermittent claudication both involve repeated periods of I-R, very few studies have focused on connecting these two phenomena. Intermittent claudication itself as a trigger of conditioning has also received little attention. In this context, a recent report points to a role for ER stress response in cardioprotection against reperfusion injury in IP20).
Supervised exercise is considered an effective first-line treatment for PAD with claudication21) and it is recommended by international guidelines as the standard of care22). It has been suggested that the physiological, metabolic, and mechanical alterations that occur during the period of exercise presumably cause an adaptive response that at the end reduces claudication symptoms23). In particular, it has been suggested that the adaptive response not only ameliorates vascular perfusion but also reduces the skeletal muscle damage that in part derives from I-R injury23). Recent studies have demonstrated that physical exercise also modifies gene expression toward an anti-inflammatory and an antiatherosclerotic pattern in circulating cells of healthy subjects24) and of PAD patients with intermittent claudication25).
Aim
This study evaluated whether a short course of supervised physical training, characterized by repeated episodes of I-R in PAD patients with intermittent claudication, reduces systemic oxidative stress and makes peripheral blood mononuclear cells (PBMCs) more resistant to I-R injuries by reducing cytotoxicity and oxidative stress through inducing an adaptative response of UPR and Nrf2 pathway expression. We also examined the effect of repeated episodes of I-R on PBMCs from PAD patients, used in this case as surrogate cells to mimic the hypoxic environment present in the skeletal muscle cells during physical activity. Finally, we assessed the requirement of UPR and Nrf2 pathways for promoting adaptation and survival to I-R in PBMCs, using specific inhibitors.
Methods
Patients
The hospital ethics committee (Azienda Ospedaliera Universitaria Integrata Verona, AOUI) in accordance with the ethical standards of the Declaration of Helsinki approved the study (Protocol number 1538), and informed written consent was obtained from all the patients before their enrolment.
24 consecutive outpatients routinely afferent to the clinical and training program offered by the Angiology Section of AOUI and meeting the following inclusion criteria were considered: age between 50 and 85 years, PAD with intermittent claudication (Ⅱ stage of Leriche Fontaine classification) diagnosed by anamnesis, clinical exam, Ankle Brachial Index (ABI) measurement (between 0.5 and 0.9 in the symptomatic leg) and complete ColorDoppler ultrasound of lower limbs that confirms extensive atherosclerotic lesions, control of dyslipidemia, diabetes, and hypertension according to current guidelines. The patients discontinued smoking from at least 6 months and no changes in their usual therapy occurred.
The exclusion criteria were: advanced diabetic microvascular disease with peripheral neuropathy (diagnosed on bases of clinical symptoms and Semmes-Weinstein monofilament test), angina, heart infarction or stroke in the 6 months before enrolment, cardiac dysfunction (FE < 40%), revascularization procedure in the 6 months before enrolment, renal failure (creatinine > 1.5 mg/dL), respiratory disease with reduced physical performance, contraindication to physical activity (e.g. orthopedic/neurological disease). The control group comprised age-, sex-, and smoking habit-matched subjects randomly selected from the general population26, 27).
Walking Ability and Physical Training
Maximal walking distance (MWD) defined as the point at which patient could no longer tolerate increase in the leg pain during walking, and time to the onset of claudication (pain-free walking distance, PFWD) were measured before and at the end of the physical training program. MWD and PFWD were determined by means of a treadmill test. Each patient underwent a pre-test on treadmill to verify the ability to walk at speed and slope defined in our protocol (3.2 km/h; slope 10%), according to TASC Ⅱ indications28). All patients underwent the standardized training period of 21 sessions as described29, 30). Each exercise session included: 30 min of aerobic exercise to stimulate proprioception and respiratory strength; treadmill exercise for 50 min; the exercise was prolonged till the onset of leg pain and then interrupted till resolution of symptoms; subsequently the patient restarted with the exercise (according with TASC Ⅱ prescriptions),28); the session was concluded with 20 min of exercise on cyclette without resistance.
Blood Samples and PBMC Isolation
Venous blood samples were obtained from each subject after 12 hour of fasting, at the start and at the end of the supervised physical program. To explore the effect of supervised exercise also on acute response to I-R, venous blood samples were obtained before, 30 and 120 min after the end of treadmill test at the beginning and at the end of the program. Blood was collected from each subject and drawn into pyrogen-free blood collection tubes. Multiple aliquots of plasma were placed into sterile 1-mL screw-capped polypropylene vials, containing the phenolic antioxidant 2,6-di-tert-butyl-4-methylphenol (10 mM; Sigma, Milan, Italy) to inhibit lipid peroxidation, and stored at −80°C. Samples were kept frozen for no longer than 6 months, with an average of 3 months. The samples were frozen and thawed only once. PBMCs were isolated as previously described31). PBMCs from patients were cultured in RPMI 1640 with L-glutamine (GIBCO), as described32).
Systemic and Cellular Markers of Oxidative Stress
Plasma GSH was analyzed using high-performance liquid chromatography (HPLC) with fluorescence detection of 7-fluorobenzo-2-oxa-1, 3-diazol-4-sulfonic acid at excitation 385 nm and emission 515 nm as previously described32). Plasma malondialdehyde (MDA) was measured using HPLC with mass spectrometer detection according to the method proposed by Mao et al.33). Intracellular ROS production was quantified through the oxidation of 2′,7′-dichlorofluorescin diacetate, as previously described34).
Cytotoxicity Evaluation in PBMCs
Cell apoptosis was evaluated by Annexin V/propidium iodide double staining assay. The cells were incubated at room temperature for 20 min in the dark and analyzed by flow cytometry. The number of each type of cells was expressed as percentages of the number of total stained cells.
LDH leakage assay was evaluated after collecting the culture medium and the cells by the Pierce LDH cytotoxicity assay kit. The cells were first sonicated to ensure the cell membrane broke down to release the total amount of LDH; subsequently, centrifugation (1,000 × g for 15 min) to clear up the cell sample was undertaken. LDH leakage was estimated from the ratio between the LDH activity in the culture medium and that of the whole cell content.
The requirement of UPR and Nrf2 pathways for promoting adaptation and survival to I-R was assessed using the following pharmacologic tools, at a concentration ranging from 1 to 3 µM: GSK compound 39 (Merck Millipore, Darmstadt, Germany), 4µ8c (Sigma Aldrich, Milan, Italy) and trigonelline (Sigma Aldrich, Milan, Italy) respectively for PERK, IRE1, and Nrf2 inhibition.
Oxidative Stress, Cytotoxicity, UPR, and Nrf2 Expression in PBMCs from PAD Patients Subjected to Repeated I-R
For I-R experiments, the EVOS FL Auto Imaging System (Thermofisher, Invitrogen) equipped with the EVOS Onstage Incubator was used, according to manufacturer's instructions. This system provides an environmental chamber allowing for the precise control of temperature, humidity, and gases (N2, CO2, and O2), and hypoxia can be monitored by long-term fluorescence live-cell imaging, using Invitrogen™ Image-iT™ Hypoxia Reagent (NucBlue Live Ready Probes Reagent).
PBMCs were cultured overnight in RPMI 1640 with L-glutamine at 37°C in an incubator set at normoxic conditions (20% O2). Then PBMCs were placed on the EVOS FL Auto Imaging System and incubated for 60 min to allow the system to reach the required temperature (37°C), humidity (> 80%) and CO2 level (5%) at normoxic conditions (20% O2). Under normoxic conditions, there was no signal from the ImageiT Hypoxia Reagent, but in response to the decrease in oxygen levels the signal from the Image-iT Hypoxia Reagent increased with nearly all the cells being hypoxic after 60 min at 5% O2 levels. The signal from Image-iT Hypoxia Reagent was reversible and when oxygen levels returned to normal, the signal decreased back to baseline. After reaching hypoxic conditions, PBMCs were subjected to multiple (5) periods (60 min) of ischemia followed by reperfusion (60 min) and oxidative stress markers, cytotoxicity, and UPR and Nrf2 expression were evaluated.
Endotoxin contamination of cell was routinely excluded with the chromogenic Limulus amoebocyte lysate assay.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated with RNEasy Mini Kit (Qiagen, Hilden, Germany). The concentration and quality of RNA were evaluated using the RNA 6000 Nano LabChip Kit (Agilent 2100 Bioer, Agilent Technologies Inc., Santa Clara, CA, USA). Reverse transcription of total RNA was carried out using IScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's recommendations. Reverse transcription was performed using the IScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The relative mRNA expression levels of PERK, ATF6, IRE1, CHOP, Nrf2, HO-1, and GCLC were performed in triplicate using the QuantiTect Primer Assay and QuantiTect SYBR Green PCR Kit (Qiagen) on the MyiQ Thermal Cycler (Bio-Rad). QuantiTect Hs-ACTB Assay (Qiagen) was used as normalizer. Normalized gene expression levels are given as the ratio between the mean value for the target gene and that for the β-actin in each sample.
Nuclear Assay of the Transcription Factors ATF4, XBP1, CHOP, and Nrf2
Nuclear ATF4, Nrf2, and CHOP were measured using sandwich enzyme-linked immunosorbent assay (ELISA) kits (LifeSpan BioScience, Inc/Seattle, USA) following manufacturer's instructions. XBP1 was measured using sandwich chemiluminescent immunoassay kit (LifeSpan BioScience, Inc/Seattle, USA) following manufacturer's instructions. Nuclear extracts were prepared using Nuclear Extraction Kit (Cayman, Ann Arbor, USA).
Statistical Analysis
Data are expressed as mean ± SD values if normally distributed. Differences between groups were analyzed by a two-tailed paired and unpaired Student's t-test and by one- or two-way analysis of variance for repeated measures followed by the post hoc Tukey test for multiple comparisons. Relationship between variables was assessed by linear regression. A probability value (p) of 0.05 was considered to be statistically significant. All data were analyzed with SPSS (IBM Corp. SPSS Statistic Version 20).
Results
Patient Characteristics and Walking Ability
The 24 PAD patients with intermittent claudication were 18 males and 6 females, with a mean age of 71.8 ± 8.2 years. According to inclusion criteria, all the patients were ex-smokers and presented an optimal control of dyslipidemia, hypertension, and diabetes (Table 1). The anthropometric characteristics, blood pressure values, lipid profile, and HbA1c were similar at beginning and at the end of the study. Table 1 also shows that supervised physical training significantly decreased plasma C reactive protein (CRP), (p < 0.01) and increased both MWD and PWFD (p < 0.001). On the contrary, ABI did not vary. The age-matched control group consisted of 30 subjects (24 males and 6 females) with a mean age of 67.8 ± 6.6 years.
Table 1. Anthropometric characteristics, blood pressure values, laboratory parameters and walking ability at the beginning and at the end of the study.
| Start | End | P | |
|---|---|---|---|
| n = 24 | n = 24 | ||
| Age (years) | 71.8 ± 8.2 | ||
| Sex (M/F) | 18/6 | ||
| Past smokers | 24/24 | 24/24 | |
| Waist circumference (cm) | 96.1 ± 11.8 | 95.3 ± 12.2 | NS |
| BMI (kg/m2) | 24.6 ± 4.8 | 24.1 ± 4.3 | NS |
| SBP (mmHg) | 128.6 ± 11.7 | 125.2 ± 12.3 | NS |
| DBP (mmHg) | 80.1 ± 7.5 | 81.3 ± 6.6 | NS |
| ABI | 0.63 ± 0.10 | 0.68 ± 0.12 | NS |
| Total cholesterol (mg/dL) | 147.9 ± 17.6 | 146.3 ± 18.3 | NS |
| LDL cholesterol (mg/dL) | 82.9 ± 13.9 | 86.7 ± 11.3 | NS |
| HDL cholesterol (mg/dL) | 36.7 ± 8.4 | 38.3 ± 8.2 | NS |
| Triglycerides (mg/dL) | 121.3 ± 24.4 | 115.8.3 ± 19.2 | NS |
| HbA1C (%) | 6.1 ± 0.3 | 6.0 ± 0.5 | NS |
| Hs-CRP (mg/L) | 2.7 ± 0.8 | 1.6 ± 0.6 | < 0.01 |
| PFWD (m) | 192.3 ± 106.2 | 359.3 ± 123.7 | < 0.001 |
| MWD (m) | 372.8 ± 133.8 | 590.8 ± 149.5 | < 0.001 |
LEGEND: BMI = body mass index; hs-CRP = high sensitivity C reactive protein; SBP = systolic blood pressure; DBP = diastolic blood pressure; ABI = ankle brachial index; PFWD = pain-free walking distance; MWD = maximal walking distance. Data are expressed as mean ± SD.
PBMC Cytotoxicity
To evaluate the effect of supervised physical training on PBMC cytotoxicity, LDH leakage and % apoptosis of cultured PBMCs were examined. The 21 sessions of physical training determined a substantial fall in LDH leakage of PBMCs (p < 0.01); in addition, at the end of the training there was a significant drop of % apoptotic PBMCs when compared with the start (p < 0.01) (Fig. 1a). The variations of LDH leakage resulted inversely correlated with those of MWD (r = −0.70, p < 0.001) (Fig. 1b).
Fig. 1.

Effect of supervised physical training on cytotoxicity in PBMCs derived from PAD patients, and correlation between the changes (delta) of maximal walking distance (MWD) with those of LDH leakage. (a) LDH leakage and apoptosis of PBMCs, (b) correlation between the changes of MWD with those of LDH leakage. Data are expressed as mean ± SD; *p < 0.01 vs start.
PBMC and Systemic Oxidative Stress
Supervised physical training was associated with a significant fall in ROS generation in PBMCs (p < 0.01) as well as a reduction in plasma MDA (p < 0.01) and an increment in plasma GSH (p < 0.01). These results are shown in Fig. 2 (a–b). At the end of the physical course, plasma MDA and GSH levels were similar to those found in the age-matched controls (respectively MDA: 1.3 ± 0.41 µmol/L vs 1.42 ± 0.51 µmol/L p = ns; GSH: 3.84 ± 0.91 µmol/L vs 3.42 ± 0.81 µmol/L p = ns). There was an inverse correlation between the changes of plasma MDA and those of MWD (r = −0.65, p = 0.001), while the changes of plasma GSH directly correlated with those of MWD (r =0.60, p =0.002), (Fig. 2 c–d). In addition, there was a positive association between the variations of plasma MDA and those of ROS in PBMCs (r = 0.62, p < 0.001) (data not shown).
Fig. 2.
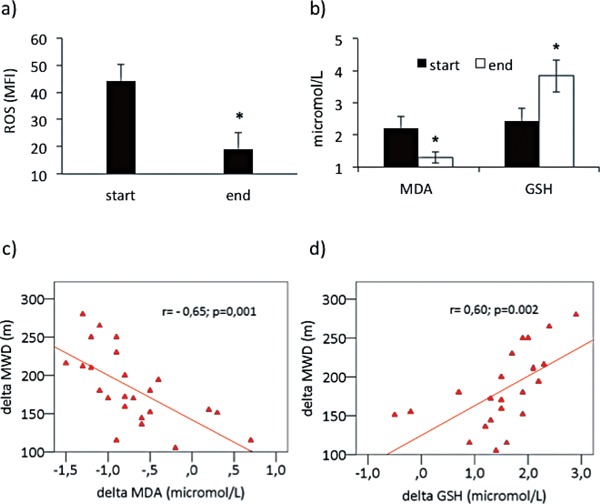
Effect of supervised physical training on reactive oxygen species (ROS) generation in PBMCs derived from PAD patients and on plasma malondialdehyde (MDA) and glutathione (GSH), and correlations between the changes (delta) of maximal walking distance (MWD) with those of MDA and GSH. (a) Reactive oxygen species (ROS) generation in PBMCs, (b) plasma MDA and GSH concentrations, c) correlation between changes of MWD and those of MDA, d) correlation between changes of MWD and those of GSH. Data are expressed as mean ± SD; *p < 0.01 vs start.
To explore the effect of supervised exercise on acute response to I-R, ROS generation in PBMCs and plasma MDA and GSH were also measured at 30 min and at 120 min after the end of treadmill test, at start and at the end of the physical training. At the beginning there was a significant increase (p < 0.01) of ROS generation and MDA that peaked at 30 min and persisted until 120 min. At the end of physical training the behavior of MDA and ROS at 30 and 120 min after the end of treadmill test was similar, but at significantly lower levels (p < 0.01). In contrast, GSH did not vary (Fig. 3a–c).
Fig. 3.
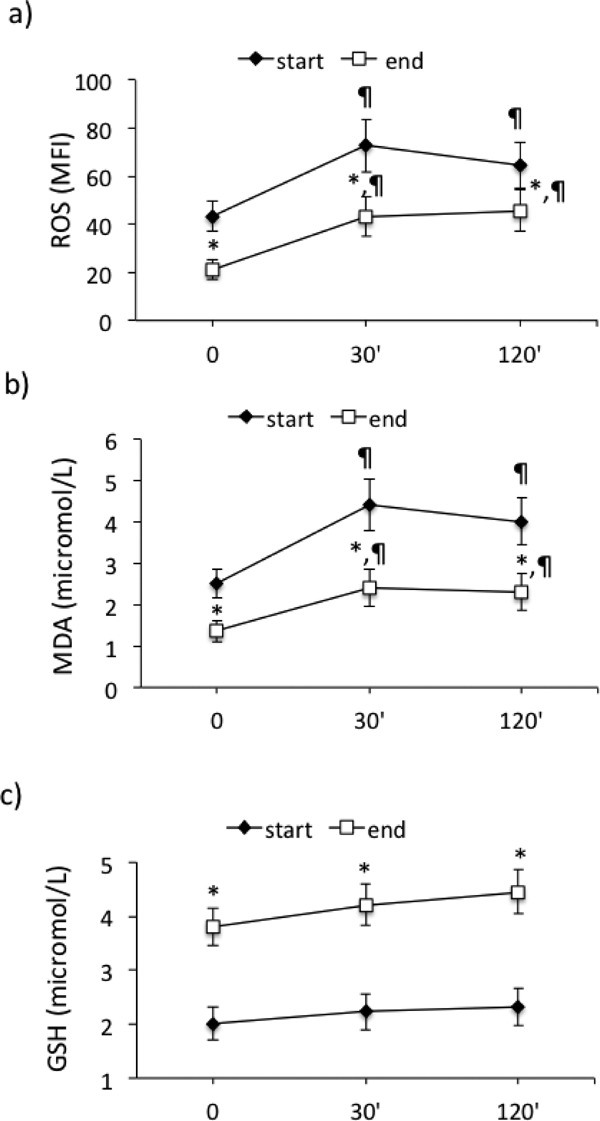
Effect of supervised physical training on acute response to ischemia-reperfusion (treadmill test) on (a) reactive oxygen species (ROS) generation in PBMCs, (b) malondialdehyde (MDA), and (c) glutathione (GSH) plasma concentrations, at the start and at the end of the supervised physical training. *p < 0.01 vs start; ¶p < 0.01 vs T0.
UPR and Nrf2 Pathway Gene Expression in PBMCs Derived from Patients
We evaluated UPR and Nrf2 pathway gene expression in PBMCs from PAD patients at the start and at the end of the physical training. Our results show a significant rise in PERK (p < 0.01) and IRE1 (p < 0.01) mRNA both basal and in response to the treadmill test (Fig. 4a), while ATF6 and CHOP mRNA did not vary (data not shown). In addition, there was a significant increase in Nrf2 (p < 0.01), HO-1 (p < 0.01), and GCLC (p < 0.01) mRNA (Fig. 4b) at the end of the physical training. Likewise, there was a significant increment in nuclear ATF4, XBP1, and Nrf2 at the end of the physical training (p < 0.01), (Fig. 4c). Basal changes of PERK and IRE1 were correlated with those of LDH leakage in PBMCs (respectively r = −0.59, p = 0.002) (Fig. 5a) and r = −0.61, p < 0.001 (data not shown). The variations of Nrf2 significantly correlated with those of ROS in PBMCs (r = −0.69, p < 0.001) (Fig. 5b).
Fig. 4.
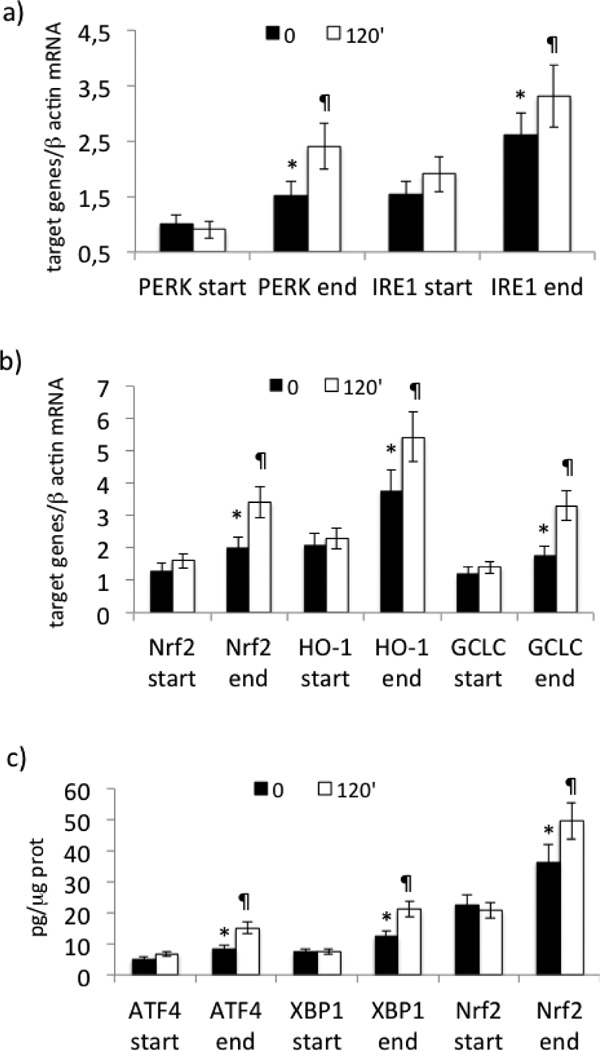
Effect of supervised physical training on UPR and Nrf2 pathway gene expression in PBMCs derived from PAD patients. (a) mRNA expression of PERK and IRE1, (b) mRNA expression of Nrf2, HO-1, and GCLC, (c) nuclear ATF4, XBP1, and Nrf2 concentrations. mRNA was analyzed by quantitative real-time PCR; normalized gene expression levels are given as the ratio between the mean value for the target gene and β-actin in each sample. Data are expressed as mean ± SD; *p < 0.01 vs start, ¶p < 0.01 vs T0.
Fig. 5.
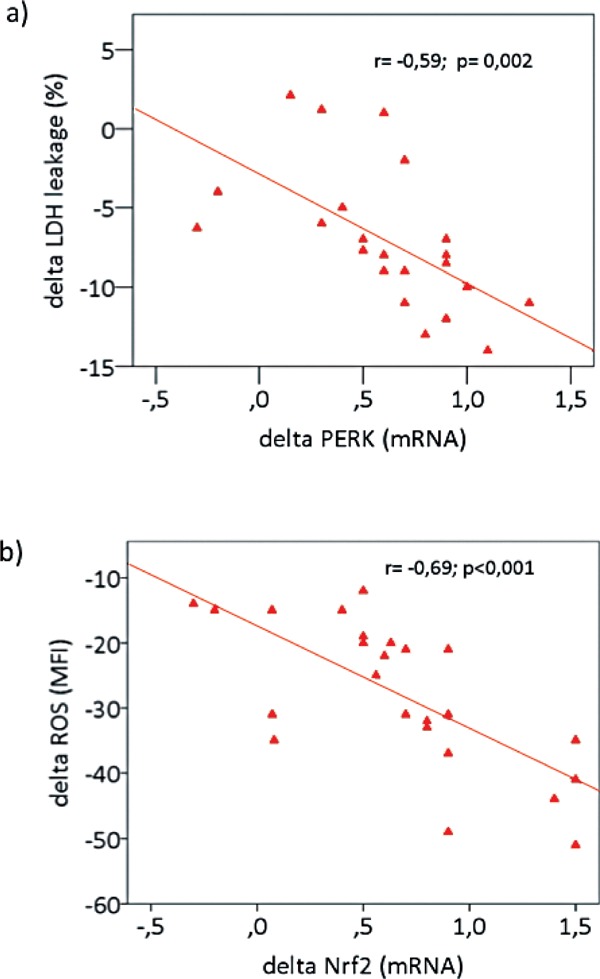
Correlations between changes (delta) in PERK with those of LDH leakage and changes in Nrf2 and reactive oxygen species (ROS) in PBMCs. a) Correlation between changes in PERK mRNA with those of LDH leakage (%). b) Correlation between changes in Nrf2 mRNA with those of ROS.
UPR and Nrf2 Pathway Gene Expression in PBMCs Derived from PAD Patients Subjected to Repeated Cycles of I-R
To understand whether physical training, characterized by multiple I-R, may act as a trigger of IP we subjected PBMCs to multiple (5) cycles of I-R. Our results show a significant augmentation of PERK (p < 0.01), IRE1 (p < 0.01), and Nrf2 mRNA expression (Fig. 6a) at the end of the 5th cycle of I-R when compared with the 1st. There was no variation of ATF6 (data not shown). These variations of UPR and of Nrf2 expression were associated with a concomitant rise in nuclear ATF4 (p < 0.01), XBP1 (p < 0.01), and Nrf2 (p < 0.01), as shown in Fig. 6b.
Fig. 6.
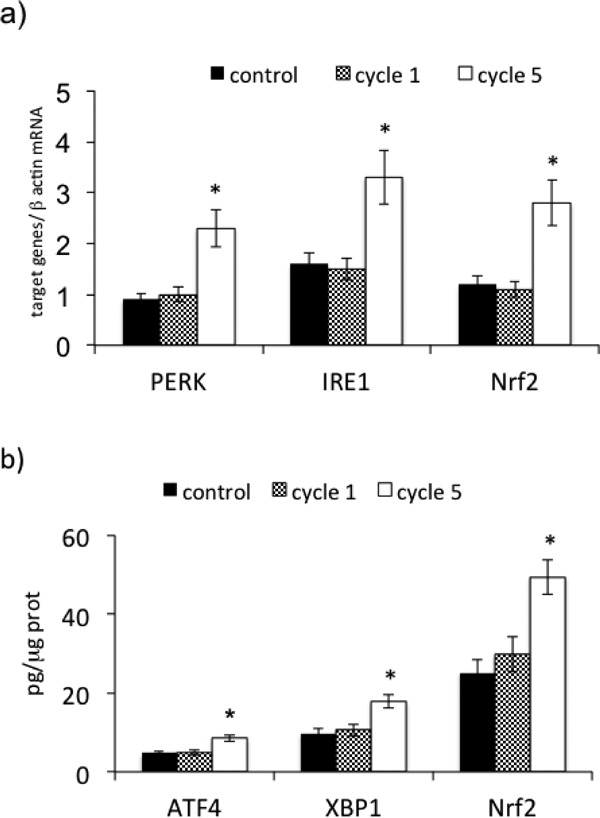
Effect of multiple (5) cycles of ischemia-reperfusion on PERK, IRE1, and Nrf2 gene expression in PBMCs derived from PAD patients. (a) mRNA expression of PERK, IRE1, and Nrf2; (b) nuclear ATF4, XBP1, and Nrf2 concentrations. mRNA was analyzed by quantitative real-time PCR; normalized gene expression levels are given as the ratio between the mean value for the target gene and β-actin in each sample. Data are expressed as mean ± SD; *p < 0.01 vs control.
Oxidative Stress and Cytotoxicity in PBMCs from PAD Patients Subjected to Repeated Cycles of I-R
When PBMCs were subjected to 5 subsequent cycles of I-R, a significant increase in ROS (p < 0.01) and in MDA (p < 0.01) in culture media was observed after the 1st cycle of I-R that was almost abolished after the 5th cycle of I-R. On the contrary, GSH in culture media was significantly reduced (p < 0.01) after the 1st cycle of I-R, returning to the basal levels after the 5th cycle of I-R (Fig. 7a–b).
Fig. 7.
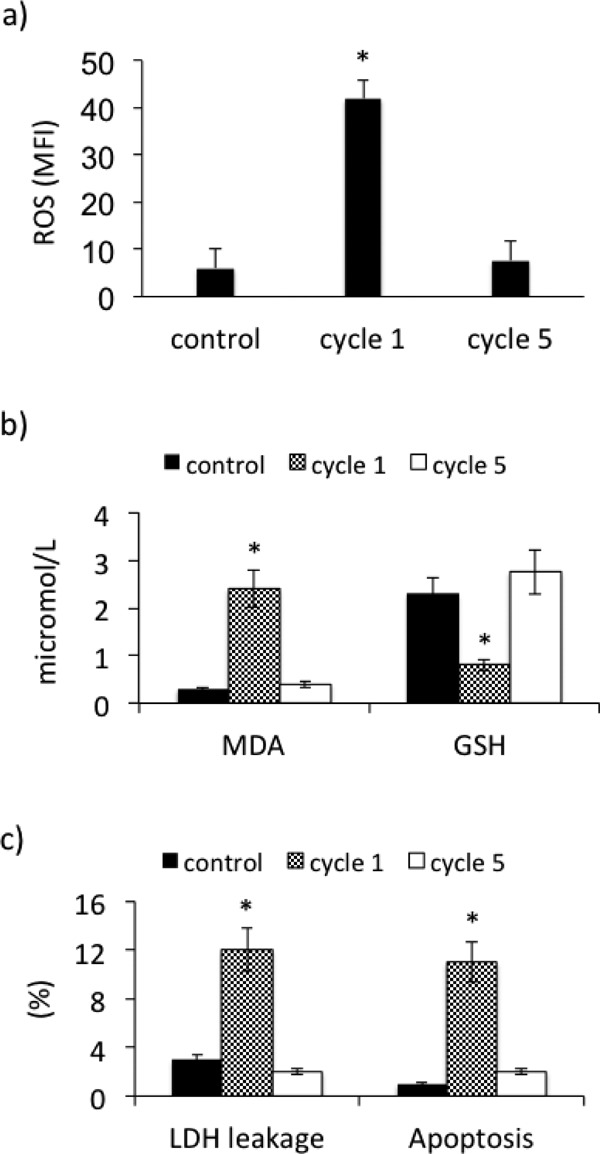
Oxidative stress and cytotoxicity in PBMCs derived from PAD patients submitted to multiple (5) cycles of ischemia-reperfusion. (a) reactive oxygen species formation (ROS), (b) malondialdehyde (MDA) and glutathione (GSH) concentrations in culture media, (c) LDH leakage and apoptosis. Data are expressed as mean ± SD; *p < 0.01 vs control.
As for cytotoxicity, the significant increase in LDH leakage (p < 0.01) and in % apoptotic cells (p < 0.01) found after the first I-R was almost abolished after the 5th cycle (Fig. 7c).
The requirement of PERK, IRE1, and Nrf2 pathways for survival signaling was assessed using the PERK inhibitor GSK compound 39, the IRE1 inhibitor 4µ8c, and the Nrf2 inhibitor trigonelline as pharmacological tools. PERK and Nrf2 inhibitors significantly reduced the positive effects of repeated episodes of I-R on LDH leakage and on % apoptosis of PBMCs (p < 0.01). The effect of IRE1 inhibitor was less evident (p < 0.05) (Fig. 8a–b).
Fig. 8.
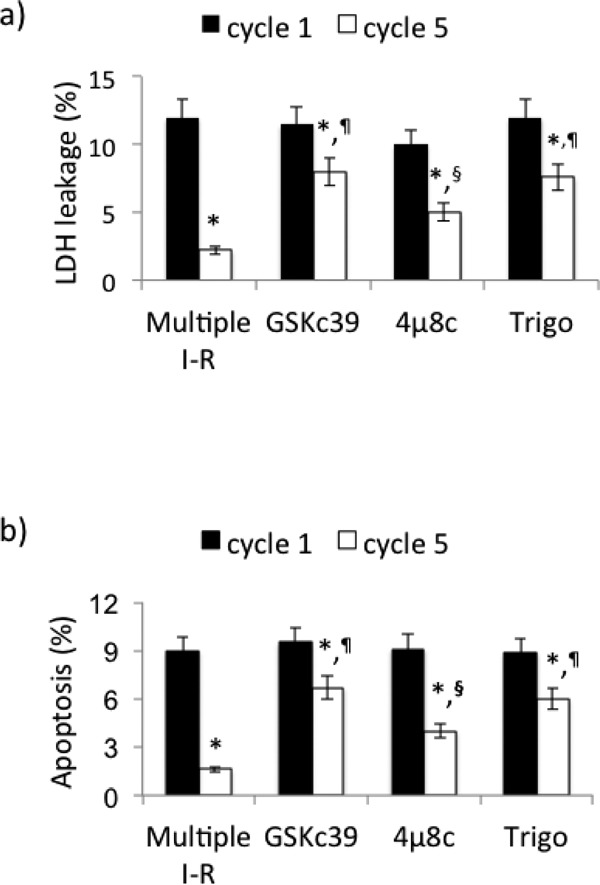
Requirement of PERK, IRE1, and Nrf2 for PBMC survival after multiple (5) cycles of I-R. (a) LDH leakage, (b) apoptosis. Data are expressed as mean ± SD; *p < 0.01 vs cycle 1. ¶p < 0.01 vs multiple I-R; §p < 0.05 vs multiple I-R; GSKc39 = GSK compound 39, trigo = trigonelline.
Discussion
The results of this study show that a short period of supervised physical activity in patients with PAD and intermittent claudication significantly increased both MWD and PFWD. Our data are in agreement with the results of Andreozzi et al.29) showing that a short course of supervised physical training is an effective tool for the treatment of intermittent claudication, providing the same improvements as the longer physical training.
In our study, supervised physical training was associated with a substantial decline in LDH leakage and in apoptosis of PBMCs derived from the patients. Furthermore, there was also a substantial decline in hs-CRP and in systemic and PBMCs oxidative stress that was paralleled by an increase in GSH, the main endogenous antioxidant that has been reported to play a key protective role in skeletal muscle35). Our results in PBMCs are in line with the reported evidence of increased oxidative stress and myocyte injury in muscle biopsy of PAD patients1, 2). Taken together these results suggest that the adaptative response induced by physical training characterized by repeated episodes of I-R may modify the cells toward a “resistant phenotype.” Though we cannot draw any definite conclusion using these results, we are tempted to speculate that the reduction in oxidative stress may have contributed to allow adaptations necessary to PBMCs' survival. In this context, our results show that physical training almost abolished the systemic and PBMCs oxidative stress induced by maximal exercise, and significantly increased GSH. Although increased levels of MDA36) and decreased levels of antioxidants37) have been shown after a treadmill test in PAD patients with intermittent claudication, our results for the first time show that supervised physical training counteracts the exacerbation of oxidative stress induced by maximal exercise. This result may have also contributed to the increased walking distance of our patients, since inhibition of oxidative stress in PAD patients with intermittent claudication has been reported to be associated with MWD improvement38). The fact that the variations of LDH leakage and MDA negatively correlated while those of GSH directly correlated with the changes of MWD may support this hypothesis.
It has been previously demonstrated that briefly exposing the brain to hypoxia in mice (i.e., hypoxic preconditioning) prior to transient middle cerebral artery occlusion reduces infarct volume, blood–brain barrier disruption, leukocyte migration, and up-regulates transcription of some genes not only in ischemic brain but also in circulating cells39). Hence it has been hypothesized that such an hypoxia-induced up-regulation of genes in peripheral cells is required for hypoxic preconditioning-induced ischemic tolerance, indicating that the process is a systemic stimulus39). A similar interplay between cells directly or not directly subjected to ischemia may also explain the protective effects of the so called “remote” preconditioning observed in both clinical and preclinical studies40–43). So one hypothesis in our patients may be that repeated episodes of I-R in the legs during the course of physical training induce ER stress, which in turn triggers UPR11) and Nrf2 pathways. The UPR transmits information on the protein folding status at the ER lumen to the cytosol and nucleus to induce adaptive responses11). Furthermore, UPR increases the biogenesis of ER, augments folding and quality control mechanisms, and regulates protein translation. Thus, UPR stress sensors can integrate information about the duration and intensity of stress and determine cell fate, i.e., adaptative response or death11). In this study, we found for the first time that physical training induced a substantial increment of PERK and IRE1 mRNA expression in PBMCs after the end of the treadmill test. Furthermore, the increase of PERK and IRE1 mRNA was associated with a considerable increment in nuclear concentration of ATF4 and XBP1, confirming the activation of UPR. Our results are in line with a series of previous reports showing activation of UPR in different models of I-R13–17) and indicate that the daily treadmill training, characterized by repeated episodes of I-R, may have contributed to a survival adaptation. That variations in PERK and IRE1 mRNA after the physical course are strictly correlated with those of LDH leakage supports this conclusion.
Similarly, a further contribution to cell survival after the period of treadmill training may be related to Nrf2 pathway activation. It is known that I-R produces ROS that injure skeletal muscle cells, impair mitochondrial function, and favor cell apoptosis and death1–6). In this context, reports show that Nrf2 attenuates the injuries caused by I-R8, 9). Similar to PERK and IRE1, supervised physical training enhanced Nrf2, HO-1, and GCLC mRNA expression both basal and after treadmill test. Increased Nrf2 in the nucleus confirms the activation of Nrf2 pathway. Since the generation of ROS has been involved in skeletal muscle injuries caused by I-R1–6), reducing oxidative stress is a potential therapeutic approach to prevent I-R injuries. Here, for the first time we show that a short course of supervised physical training is associated with a substantial increment in Nrf2 and of related ARE genes in PBMCs derived from PAD patients with intermittent claudication. In these patients the daily physical activity, i.e., treadmill training, is characterized by repeated episodes of I-R, and our results, under different experimental conditions, are similar to previous findings, indicating that Nrf2 accumulates in the nucleus after I-R in cardiac44) and renal tissues45). Moreover, several compounds that activate the Nrf2 pathway have been shown to be protective against I-R damage8, 9). These observations indicate that the activation of the Nrf2/ARE pathway induced by treadmill training in patients with PAD may protect against I-R injury and be one of the potential determinants of survival adaptation. The variations of Nrf2 mRNA after the physical course are correlated with those of ROS generation in PBMCs and support this conclusion.
Although with the present data we cannot draw the conclusion that PBMC changes in PAD patients reflect myocyte damages, the results of this study strongly suggest that PBMCs may represent a promising, noninvasive, useful marker of systemic cytotoxicity and oxidative stress. The fact that physical training modified these parameters further supports this hypothesis. Taken together, the results of this study show that a short course of physical training causes a series of adaptations in circulating cells of patients with PAD that may reflect the changes in skeletal muscle of the leg directly subjected to ischemia and that may eventually contribute to reduce cellular injuries elicited by I-R.
To date there are no studies addressing the positive or negative effects of PERK/ATF4 over-expression or down-regulation during I-R injury in animal models, whereas transgenic over-expression of XBP1s in cardiomyocytes has been shown to reduce infarct size and improve heart function after in vivo I-R injury46). Hence, we cannot conclude with the present data that the reduction in cell damage, and the consequent improvement in cell survival induced by a short course of physical activity, are dependent only on I-R-induced UPR and Nrf2 activation. It is a suggestive association, and further studies are needed to confirm a cause and effect relationship. To overcome this limitation, we performed an ex vivo study to evaluate UPR and Nrf2 gene expression as well as cell damages and oxidative stress in PBMCs from PAD patients submitted to multiple consecutive episodes of I-R. In this case, PBMCs were used as surrogate of skeletal muscle cells where injuries are caused directly by ischemia. When PBMCs were subjected to multiple, relatively brief episodes of I-R, UPR and Nrf2 expression increased at the end of the 5th cycle of I-R, while oxidative stress and cytotoxicity declined after an initial rise. To the best of our knowledge, this is the first demonstration that relatively brief, repeated episodes of I-R have an antioxidant and survival role probably related to UPR and Nrf2 up-regulation. Although with the present results we cannot offer a full mechanistic explanation, one hypothesis could be that brief, repeated episodes of I-R in skeletal muscle cells may trigger hypoxic adaptation similar to IP. The fact that treadmill training has been shown to reduce skeletal muscle damage23) may support the idea that repeated brief episodes of I-R are a trigger of conditioning through UPR and Nrf2 activation. A limitation is that our data have been obtained using surrogate cells in culture experiments, and further studies are warranted to confirm the results in vivo.
Finally, the requirement of PERK, IRE1, and Nrf2 pathways for survival signaling was assessed using specific inhibitors as pharmacological tools. Inhibitors of PERK, IRE1 and Nrf2 reduced the positive effect of multiple, brief episodes of I-R on oxidative stress and cytoxicity, indicating that PERK and Nrf2 and, to a lesser extent IRE1, play a key role in the adaptative response of PBMCs submitted to I-R.
Conclusions
In this study PBMCs have been shown to be a promising non-invasive useful marker of adaptations induced by physical training in PAD patients with intermittent claudication. In particular, the results demonstrate that treadmill training causes a series of PBMC adaptations, i.e., up-regulation of UPR and Nrf2 gene expression, that may contribute to modify the cells toward a “resistant phenotype.”
Acknowledgments
This study was performed (in part) in the LURM (Laboratorio Universitario di Ricerca Medica) Research Center, University of Verona.
Conflict of Interests
All authors declare that they have no conflict of interest or financial disclosures.
Sources of Funding
This work was supported in part by grants from University of Verona (Fondo Unico per la Ricerca), Italy, and from Fondazione Cariverona, Italy.
References
- 1). Weiss DJ, Casale GP, Koutakis P, Nella AA, Swanson SA, Zhu Z, Miserlis D, Johanning JM, Pipinos II: Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease, J Transl Med, 2013; 11: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Gillani S, Cao J, Suzuki T, Hak DJ: The effect of ischemia reperfusion injury on skeletal muscle, Injury, 2012; 43: 670-675 [DOI] [PubMed] [Google Scholar]
- 3). Pipinos II, Swanson SA, Zhu Z, Nella AA, Weiss DJ, Gutti TL, McComb RD, Baxter BT, Lynch TG, Casale GP: Chronically ischemic mouse skeletal muscle exhibits myopathy in association with mitochondrial dysfunction and oxidative damage, Am J Physiol Regul Integr Comp Physiol, 2008; 295: R290-R296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Pipinos II, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, Baxter BT, Lynch TG, Dodd SL: Mitochondrial defects and oxidative damage in patients with peripheral arterial disease, Free Radic Biol Med, 2006; 41: 262-269 [DOI] [PubMed] [Google Scholar]
- 5). Pipinos II, Sharov VG, Shepard AD, Anagnostopoulos PV, Katsamouris A, Todor A, Filis KA, Sabbah HN: Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease, J Vasc Surg, 2003; 38: 827-832 [DOI] [PubMed] [Google Scholar]
- 6). Anderson JD, Epstein FH, Meyer CH, Hagspiel KD, Wang H, Berr SS, Harthun NL, Weltman A, Dimaria JM, West AM, Kramer CM: Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism, J Am Coll Cardiol, 2009; 54: 628-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Nguyen T, Sherratt PJ, Pickett CB: Regulatory mechanisms controlling gene expression mediated by the antioxidant response element, Annu Rev Pharmacol Toxicol, 2003; 43: 233-260 [DOI] [PubMed] [Google Scholar]
- 8). Calvert JW, Elston M, Nicholson CK, Susheel Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ: Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice, Circulation, 2010; 122: 11-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Ashrafian H, Czibik G, Bellahcene M, Aksentijevic D, Smith AC, Mitchell SJ, Dodd MS, Kirwan J, Byrne JJ, Ludwig C, Isackson H, Yavari A, Støttrup NB, Contractor H, Cahill TJ, Sahgal N, Ball DR, Birkler RI, Hargreaves I, Tennant DA, Land J, Lygate CA, Johannsen M, Kharbanda RK, Neubauer S, Redwood C, de Cabo R, Ahmet I, Talan M, Günther UL, Robinson AJ, Viant MR, Pollard PJ, Tyler DJ, Watkins H: Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway, Cell Metab, 2012; 15: 361-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Hetz C: The unfolded protein response controlling cell fate decisions under ER stress and beyond, Nat Rev Mol Cell Biol, 2012; 13: 89-102 [DOI] [PubMed] [Google Scholar]
- 11). Dufey E, Sepúlveda D, Rojas-Rivera D, Hetz C: Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview, Am J Physiol Cell Physiol, 2014; 307: C582-C594 [DOI] [PubMed] [Google Scholar]
- 12). Altamirano F, Wang ZV, Hill JA: Cardioprotection in ischaemia-reperfusion injury: novel mechanisms and clinical translation, J Physiol, 2015; 593: 3773-3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC: Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6, Circ Res, 2006; 98: 1186-1193 [DOI] [PubMed] [Google Scholar]
- 14). Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC: Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response, J Biol Chem, 2009; 284: 29735-29745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Brooks AC, Guo Y, Singh M, McCracken J, Xuan YT, Srivastava S, Bolli R, Bhatnagar A: Endoplasmic reticulum stress-dependent activation of ATF3 mediates the late phase of ischemic preconditioning, J Mol Cell Cardiol, 2014; 76: 138-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Szegezdi E, Duffy A, O'Mahoney ME, Logue SE, Mylotte LA, O'Brien T, Samali A: ER stress contributes to ischemia-induced cardiomyocyte apoptosis, Biochem Biophys Res Commun, 2006; 349: 1406-1411 [DOI] [PubMed] [Google Scholar]
- 17). Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC: Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes, Circ Res, 2006; 99: 275-282 [DOI] [PubMed] [Google Scholar]
- 18). Hausenloy DJ, Yellon DM: The therapeutic potential of ischemic conditioning: an update, Nat Rev Cardiol, 2011; 8: 619-629 [DOI] [PubMed] [Google Scholar]
- 19). Whittaker P, Przyklenk K: From ischemic conditioning to 'hyperconditioning': clinical phenomenon and basic science opportunity, Dose Response, 2014; 12: 650-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Grall S, Prunier-Mirebeau D, Tamareille S, Mateus V, Lamon D, Furber A, Prunier F: Endoplasmic reticulum stress pathway involvement in local and remote myocardial ischemic conditioning, Shock, 2013; 39: 433-439 [DOI] [PubMed] [Google Scholar]
- 21). Fakhry F, van de Luijtgaarden KM, Bax L, den Hoed PT, Hunink MG, Rouwet EV, Spronk S: Supervised walking therapy in patients with intermittent claudication, J Vasc Surg, 2012, 56: 1132-1142 [DOI] [PubMed] [Google Scholar]
- 22). Tendera M, Aboyans V, Bartelink ML, Aumgartner I, Clément D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Röther J, Sievert H, van Sambeek M, Zeller T: ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology, Eur Heart J, 2011; 32: 2851-2906 [DOI] [PubMed] [Google Scholar]
- 23). Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT: Exercise training for claudication, N Engl J Med, 2002; 347: 1941-1951 [DOI] [PubMed] [Google Scholar]
- 24). Radom-Aizik S, Zaldivar FP, Jr, Haddad F, Cooper DM: Impact of brief exercise on circulating monocyte gene and microRNA expression: implications for atherosclerotic vascular disease, Brain Behav Immun, 2014; 39: 121-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Dopheide JF, Scheer M, Doppler C, Obst V, Stein P, Vosseler M, Abegunewardene N, Gori T, Münzel T, Daiber A, Radsak MP, Espinola-Klein C: Change of walking distance in intermittent claudication: impact on inflammation, oxidative stress and mononuclear cells: a pilot study, Clin Res Cardiol, 2015; 104: 751-763 [DOI] [PubMed] [Google Scholar]
- 26). De Marco R, Accordini S, Antonicelli L, Bellia V, Bettin MD, Bombieri C, Bonifazi F, Bugiani M, Carosso A, Casali L, Cazzoletti L, Cerveri I, Corsico AG, Ferrari M, Fois AG, Lo Cascio V, Marcon A, Marinoni A, Olivieri M, Perbellini L, Pignatti P, Pirina P, Poli A, Rolla G, Trabetti E, Verlato G, Villani S, Zanolin ME: The Gene-Environment Interactions in Respiratory Diseases (GEIRD) Project, Int Arch Allergy Immunol, 2010; 152: 255-263 [DOI] [PubMed] [Google Scholar]
- 27). Fratta Pasini A, Ferrari M, Stranieri C, Vallerio P, Mozzini C, Garbin U, Zambon G, Cominacini L: Nrf2 expression is increased in peripheral blood mononuclear cells derived from mild-moderate ex-smoker COPD patients with persistent oxidative stress, Int J COPD, 2016; 11: 1733-1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG: TASC Ⅱ Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease, J Vasc Surg, 2007; 45 Suppl S: S5-67 [DOI] [PubMed] [Google Scholar]
- 29). Andreozzi GM, Martini R, Laudani R, Salimistraro G, Deinite G: Effectiveness and costs of a short-course supervised training program in claudicants: proposal for a shared protocol with aerobic working load, Int Angiol, 2008; 27: 401-407 [PubMed] [Google Scholar]
- 30). Andreozzi GM, Kalodiki E, L Gašpar L, Martini R, Minar E, Angelides N, Nicolaides AN, Novo S, Visonà A, Prior M, Arosio E, Hussein EA, Poredos P, Antignani PL, Avram R, Roztocil K, Stvrtinova V, Kozak M, Vacula I: Consensus Document on Intermittent Claudication from the Central European Vascular Forum (C.E.V.F.)-3rd revision (2013), Int Angiol, 2014; 33: 329-347 [PubMed] [Google Scholar]
- 31). Fratta Pasini A, Anselmi M, Garbin U, Franchi E, Stranieri C, Nava MC, Boccioletti V, Vassanelli C, Cominacini L: Enhanced levels of oxidized low-density lipoprotein prime monocytes to cytokine overproduction via up-regulation of CD14 and toll-like receptor 4 in unstable angina, Arterioscler Thromb Vasc Biol, 2007, 27: 1991-1997 [DOI] [PubMed] [Google Scholar]
- 32). Garbin U, Fratta Pasini A, Stranieri C, Cominacini M, Pasini A, Manfro S, Lugoboni F, Mozzini C, Guidi G, Faccini G, Cominacini L: Cigarette smoking blocks the protective expression of Nrf2/ARE pathway in peripheral mononuclear cells of young heavy smokers favouring inflammation, PLoS One, 2009; 4: e8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Mao J, Zhang H, Luo J, Li L, Zhao R, Zhang R, Liu G: New method for HPLC separation and fluorescence detection of malonaldehyde in normal human plasma, J Chromatogr B Analyt Technol Biomed Life Sci, 2006; 17: 832-838 [DOI] [PubMed] [Google Scholar]
- 34). Cominacini L, Fratta Pasini A, Garbin U, Davoli A, Tosetti ML, Campagnola M: Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappa B through an increased production of intracellular reactive oxygen species, J Biol Chem, 2000; 275: 12633-12638 [DOI] [PubMed] [Google Scholar]
- 35). Sirsjo A, Kagedal B, Arstrand K, Lewis DH, Nylander G, Gidlöf A: Altered glutathione levels in ischemic and postischemic skeletal muscle: difference between severe and moderate ischemic insult, J Trauma, 1996; 41: 123-128 [DOI] [PubMed] [Google Scholar]
- 36). Hickman P, Harrison DK, Hill A, McLaren M, Tamei H, McCollum PT, Belch JJ: Exercise in patients with intermittent claudication results in the generation of oxygen derived free radicals and endothelial damage, Adv Exp Med Biol, 1994; 361: 565-570 [DOI] [PubMed] [Google Scholar]
- 37). Turton EP, Coughlin PA, Kester RC, Scott DJ: Exercise training reduces the acute inflammatory response assciated with claudication, Eur J Vasc Endovasc Surg, 2002; 23: 309-316 [DOI] [PubMed] [Google Scholar]
- 38). Loffredo L, Pignatelli P, Cangemi R, Andreozzi P, Panico MA, Meloni V, Violi F: Imbalance between nitric oxide generation and oxidative stress in patients with peripheral arterial disease: effect of an antioxidant treatment, J Vasc Surg, 2006; 44: 525-530 [DOI] [PubMed] [Google Scholar]
- 39). Stowe AM, Wacker BK, Cravens PD, Perfater JL, Li MK, Hu R, Angela B, Freie AB, Stüve O, Gidday JM: CCL2 upregulation triggers hypoxic preconditioning-induced protection from stroke, J Nueroinflamm, 2012; 9: 33-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Dave KR, Saul I, Prado R, Busto R, Perez-Pinzon MA: Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest, Neurosci Lett, 2006; 404: 170-175 [DOI] [PubMed] [Google Scholar]
- 41). Ren C, Gao X, Steinberg GK, Zhao H: Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning, Neuroscience, 2008; 151: 1099-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Wenwu Z, Debing Z, Renwei C, Jian L, Guangxian Y, Pingbo L, Xinmin Z: Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants, Pediatr Cardiol, 2009; 31: 22-29 [DOI] [PubMed] [Google Scholar]
- 43). Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, Boyle JR, Varty K, Kharbanda RK, Dutka DP, Gaunt ME: Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial, Circulation, 2007; 116: I98-I105 [DOI] [PubMed] [Google Scholar]
- 44). Anedda A, López-Bernardo E, Acosta-Iborra B, Saadeh Suleiman M, Landázuri MO, Cadenas S: The transcription factor Nrf2 promotes survival by enhancing the expression of uncoupling protein 3 under conditions of oxidative stress, Free Radic Biol Med, 2013; 61: 395-407 [DOI] [PubMed] [Google Scholar]
- 45). Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O'Farrelly C, Rabb H, Taylor CT: Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury, FASEB J, 2006; 20: 2624-2626 [DOI] [PubMed] [Google Scholar]
- 46). Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, Criollo A, Luo X, Tan W, Jiang N, Lehrman MA, Rothermel BA, Lee AH, Lavandero S, Mammen PP, Ferdous A, Gillette TG, Scherer PE, Hill JA: Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway, Cell, 2014; 156: 1179-1192 [DOI] [PMC free article] [PubMed] [Google Scholar]


