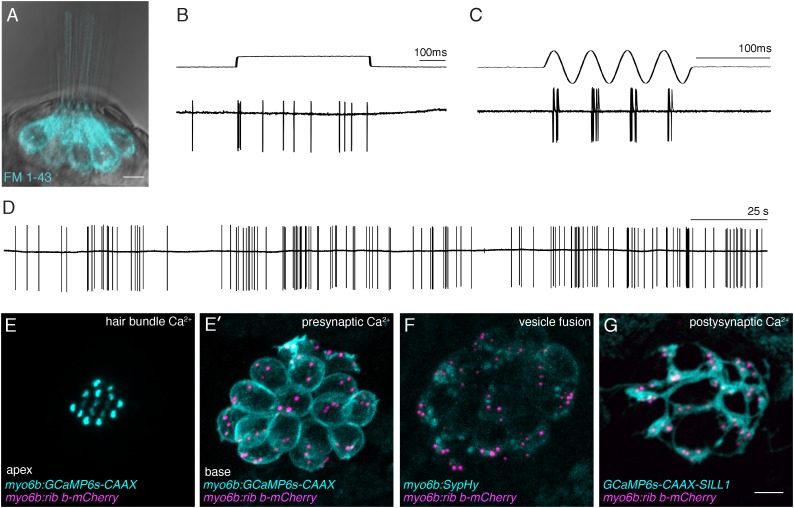
FIGURE 3.
Functional analysis of hair-cell synapses in the zebrafish lateral line. (A) In the lateral line, rapid uptake of the vital dye FM 1-43 (cyan), shown in this example, indicates mechanotransduction is intact in these hair cells. (B–D) Extracellular recordings from the afferent cell bodies in the posterior lateral-line ganglia can be used as a read-out of synapse function. During these afferent recordings, evoked spikes can be detected when innervated hair cells are stimulated along their axis of sensitivity (B,C). Step stimuli (B, anterior step shown) are useful to quantify spike number and the timing of the first spike. Sine stimuli (C, anterior-posterior sine stimulus shown) are useful to quantify the precision of spike timing within the waveform. Note that each afferent only responds to one direction of stimuli (for example the anterior but not posterior phase of the sine wave in C). Even in the absence of stimuli there is spontaneous spiking in that can be used as a read-out of synaptic function (D). (E,E’) A transgenic line expressing a membrane-localized calcium indicator GCaMP6s (cyan) can be used to detect mechanotransduction-dependent calcium influx in apical hair bundles (E) and calcium influx at synaptic ribbons (E’) when apical and basal planes are imaged respectively. (F) Transgenic fish expressing SypHy, an indicator of vesicle fusion can be used to detect presynaptic vesicle fusion at hair-cell synapses. (G) GCaMP6s can also be used to detect postsynaptic calcium activities in the afferent process beneath hair cells. All of the transgenic approaches outlined in (E–G) can be used in combination with a transgenic line that marks synaptic ribbons via a Ribeye b-mCherry fusion protein. The scale bar in (A,G) = 5 μm.