Figure 1.
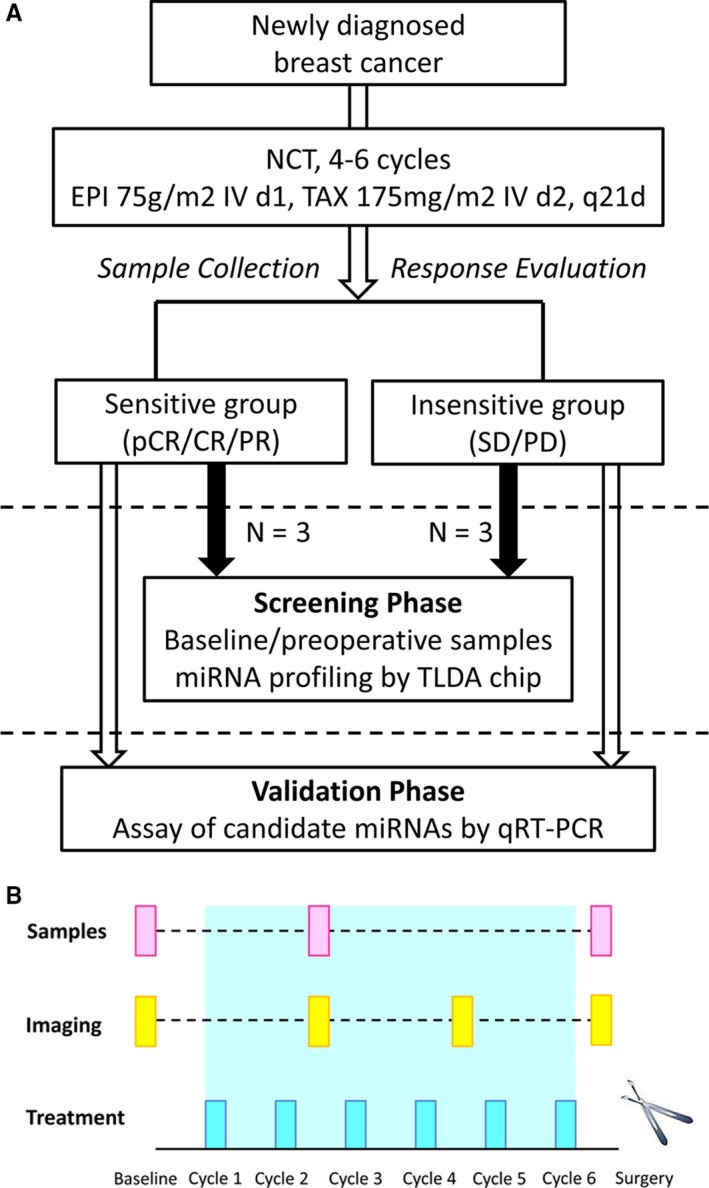
Study design and Schedule for sample collection. A, A two‐phase study was designed. In the screening phase, baseline and preoperative blood samples of selected cases from both groups were screened via microarray for candidate miRNAs whose fluctuations might reflect response. In the validation phase, the fluctuation patterns of candidate miRNAs were confirmed by qRT‐PCR using serially collected blood samples, and the association between dynamics of plasma miRNAs and chemo‐sensitivity was explored. B, For each participant, blood samples were collected at baseline, after two cycles of chemotherapy and before definitive surgery
