Abstract
Cryptococcosis is an invasive fungal infection in immunocompromised patients. The clinicopathological characteristics of cryptococcal lymphadenitis are not well known. We analyzed three cases of cryptococcal lymphadenitis and compared their characteristics with those in previous reports. Two patients were human immunodeficiency virus (HIV) carriers, and one patient was a human T-cell leukemia virus type-1 (HTLV-1) carrier. The age of the HTLV-1 carrier with cryptococcosis was much higher than that of the HIV-1 carriers. CD4-positive cell counts in peripheral blood were 5.8/µL (Case 1) and 79.9/µL (Case 2) in the HIV carriers and 3285/µL in the HTLV-1 carrier (Case 3). According to flow cytometric analysis of the lymph nodes of Cases 1, 2, and 3, 50.0%, 87.1%, and 85.9%, respectively, of the T-cells were CD3; 9.8%, 16.3%, and 75.8%, respectively, were CD4; and 35.5%, 77.3%, and 10.2%, respectively, were CD8. Cryptococcus neoformans was detected in tissue culture in all patients. Although gelatinous lesions and numerous fungal cocci were observed in the two HIV patients, the granuloma formation was small. Gelatinous formation and granuloma formation were observed in the HTLV-1 carrier. Necrosis was observed in all cases. In previous reports, granuloma formation, epithelioid cells, and necrotic lesions were observed in most cases. Most of the patients were also immunosuppressed. However, no HTLV-1 carrier was detected. In conclusion, lymphadenopathy in a HTLV-1 carrier may suggest the presence of cryptococcal lymphadenitis. The frequency of cryptococcosis in HTVL-1 carriers may increase with increase in the long-term survival rate of HTLV-1 carriers.
Keywords: Cryptococcal lymphadenitis, Immunocompromised, HTLV-1, HIV, Cryptococcosis
INTRODUCTION
Cryptococcal infection is widely known as an invasive fungal infection in immunocompromised patients. In particular, Cryptococcus neoformans can lead to a fatal fungal infection in patients with acquired immunodeficiency syndrome (AIDS) despite antifungal therapies.1 Most patients with cryptococcosis and AIDS reportedly have a CD4-positive (CD4+) T lymphocyte count <100 cells/mL in peripheral blood.1 Although cryptococcal infection involves multiple organs, cryptococcosis manifesting as lymphadenitis only is very rare.2 Only a few cases have been reported, and most of these patients were infected with human immunodeficiency virus (HIV) or had an immunocompromised status.3-9
The histological characteristics of C. neoformans infection include gelatinous and granulomatous findings.10 In addition, lesions in the early phase are more gelatinous, with numerous organisms. The inflammatory reactions to cryptococcosis have been thought to be weak in AIDS patients, resulting in poor granuloma formation.11 This phenomenon has been explained by the decreased counts of CD4+ T lymphocytes.12
Although the clinicopathological characteristics of cryptococcosis, including mainly pneumonia, meningitis, and infiltration of bone marrow, have been reported,10,11,13 those of cryptococcal lymphadenitis are not well known. In addition, the development of cryptococcal lymphadenitis in human T-cell leukemia virus type-1 (HTLV-1) carriers has not been reported. We herein report three patients with cryptococcal lymphadenitis, including an HTLV-1 carrier and two HIV carriers, and summarize the clinicopathological features of cryptococcal lymphadenitis by reviewing previously reported cases.
MATERIALS AND METHODS
Patients
Three patients were diagnosed with cryptococcal lymphadenitis in the Department of Pathology, Kurume University School of Medicine, between January 2004 and December 2015. Experienced pathologists (H.M. and K.O.) retrospectively reviewed the histologic features and we collected the clinical information.
This study was conducted in accordance with the Helsinki Declaration and was approved by the ethics review committee of Kurume University.
Histopathology and flow cytometry
Lymph node biopsies were performed in all three patients after lymphadenopathies were identified on computed tomography (CT). Sections from formalin-fixed, paraffin-embedded blocks were stained with hematoxylin and eosin and Grocott’s silver staining.
Peripheral blood samples were collected at the initial diagnosis. Flow cytometry (FCM) was used to analyze unprocessed peripheral blood and lymph node samples for CD4+ cell counts according to the institutional procedures.14-18
Identification of cryptococcal organisms.
To identify the type of cryptococcal organism, lymph node fungal culture was performed.
RESULTS
Clinical characteristics
The clinical characteristics of the three patients are shown in Table 1. Of the three patients, two were HIV carriers (Cases 1 and 2), and one was an HTLV-1 carrier (Case 3). Both HIV carriers had a high fever at the diagnosis. All three patients had elevated serum β-D glucan levels. CT showed multiple lymphadenopathies in all three patients. However, no lesions other than lymphadenopathy were detected in all cases. The CD4+ cell counts of the two HIV carriers were 5.8/µL (Case 1) and 79.9/µL (Case 2), and that of the HTLV-1 carrier (Case 3) was 3285/µL. The respective percentages of T-cells and B-cells in the lymph nodes according to the FCM analysis are shown in Table 2. The percentages of CD3-positive cells were 50.0% (Case 1), 87.1% (Case 2), and 85.9% (Case 3). The percentages of CD4-positive cells were 9.8% (Case 1), 16.3% (Case 2), and 75.8% (Case 3). The percentages of CD8-positive cells were 35.5% (Case 1), 77.3% (Case 2), and 10.2% (Case 3). The percentages of CD20-positive cells were 35.9% (Case 1), 15.8% (Case 2), and 14.4% (Case 3). Evidence of C. neoformans was present in the tissue cultures of the lymph nodes in all three patients.
Table 1. Clinical characteristics of three patients in this study.
| Case | Age | Sex | Complication | Fever | Sites of lymphadenopathy |
LDH | β-D glucan (Serum) |
CD4+ cell in PB (/mm3) |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | M | HIV carrier | + | bilateral neck | elevated | elevated | 5.8 |
| 2 | 43 | F | HIV carrier | + | systemic | elevated | elevated | 79.9 |
| 3 | 85 | F | HTLV-1carrier | - | bilateral axilla | normal | slightly elevated | 3285 |
LDH: Lactate dehydrogenase, PB: Peripheral Blood, HIV: Human Immunodeficiency Virus, HTLV-1: Human T-cell Leukemia Virus-1
Table 2. Flow cytometric analysis and pathological findings of lymph nodes.
| Case | Site | CD3 (%) |
CD4 (%) |
CD8 (%) |
CD20 (%) |
Geratinous lesion |
Granuloma formation |
Epithelioid cells (/HPF) |
Fungal load | Necrosis | Fibrosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | neck | 50.0 | 9.8 | 35.5 | 35.9 | well formed | poor | 0 | numerous | none | none |
| 2 | neck | 87.1 | 16.3 | 77.3 | 15.8 | well formed | partially formed | 0 | umerous | partially | none |
| 3 | axilla | 85.9 | 75.8 | 10.2 | 14.4 | partially formed | formed | 1-2 | focal | partially | none |
HPF: High Power Field
Pathological features
Pathological findings and images are shown in Table 2 and Figure 1. In the HIV carriers, although the gelatinous lesion was well formed and there were numerous fungal loads in the gelatinous areas, the granuloma formation and giant cells were small. However, both gelatinous formation and granuloma formation were partially observed in the lymph node, and giant cells were also observed, in the HTLV-1 carrier. In addition, necrotic lesions were observed in all cases. With Grocott’s silver stain, numerous fungal cocci were observed in the gelatinous area in the HIV-positive patients, while a small amount of fungal cocci were observed in a focal area in the HTLV-1-positive patient.
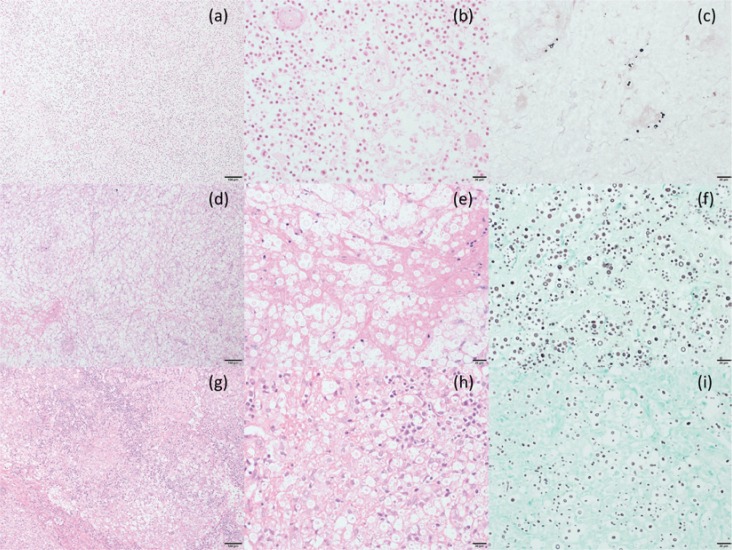
Fig. 1.
Pathological images of cases of cryptococcal lymphadenitis.
Case 1: (a)-(c), Case 2: (d)-(f), Case 3: (g)-(i).
(a), (d), (g): Hematoxylin and Eosin (HE) stain ×100, (b), (e), (h): HE stain ×400, (c), (f), (i): Grocott’s silver stain ×400
In Cases 1 and 2, although gelatinous lesions were well formed and numerous fungal loads were diffusely observed in the gelatinous areas, the granuloma formation and proportion of giant cells were small (a, b, d, e). However, both gelatinous formation and granuloma formation were partially observed in the lymph node in Case 3 (g, h). Giant cells were observed in the HTLV-1 carrier (g, h). Necrotic lesions were observed in all cases (a, b, d, e). With Grocott’s silver stain, numerous fungal cocci were observed in the gelatinous area in Cases 1 and 2 (c, f), while only few fungal cocci were observed in the focal area in Case 3 (i).
DISCUSSION
The clinical backgrounds and pathological features of the three patients with cryptococcal lymphadenitis that we described differed slightly between the HIV carriers and HTLV-1 carrier. Strongyloidiasis, tuberculosis, and leprosy are well-recognized infection complications of chronic HTLV-1 infection (HTLV-1 carriers)19; they are associated with the immunodeficiency caused by chronic HTLV-1 infection.20-27 Approximately 5-8% of patients with AIDS develop cryptococcal infection. However, the incidence of cryptococcosis has decreased since the availability of effective antiretroviral treatment.28,29 On the other hand, to our best knowledge, the present report is the first report of cryptococcal lymphadenitis in an HTLV-1 carrier.
Cryptococcal lymphadenitis is clinically associated with an immunocompromised status as well as other cryptococcal infections. Similar to previous reports, the CD4+ cell counts of the two patients with HIV infection were < 100/µL.1,30,31 Cryptococcal-specific CD4+ T-cell responses are reportedly associated with the development and disease severity of cryptococcosis.32 In addition, decreased CD4+ T-cell counts are considered to induce depressed cell-mediated immunity for cryptococcal antigen.32,33 HTLV-1, in addition to HIV, infects CD4+ cells, resulting in the development of adult T-cell leukemia/lymphoma (ATLL) in some carriers.34 Despite an increased CD4-positive cell count, the counts of normal CD4+ cells are decreased. According to the FCM analysis of the lymph node, the proportion of CD4+ cells was also lower in the present study. It might be necessary to consider not only the development of ATLL but also the onset of cryptococcal lymphadenitis when diagnosing lymphadenopathy in HTLV-1 carrier patients.
The pathological findings showed the association between the normal CD4+ cell count and granuloma formation in the present study. A previous study also reported that the histology of cryptococcal lymphadenitis showed necrotizing granulomas, as in the present patients.9
However, the granulomatous reaction and inflammatory response to a cryptococcal infection was very slight in the HTLV-1 carrier, unlike the HIV-1 carriers. In contrast, a giant cell reaction was observed in the HTLV-1 carrier, similar to that observed previously with cryptococcal lymphadenitis.3,9 Therefore, the clues for the diagnosis of cryptococcal lymphadenitis might be necrotizing granulomatous formation, gelatinous formation, and giant cell reaction.
Table 3 summarizes the clinicopathological features of previously reported cases with cryptococcal lymphadenitis.4-6,9,35 Of the 19 cases for which the clinicopathological findings of cryptococcal lymphadenitis were reported, there were 13 immunocompromised patients (10 patients with, 2 patients post-kidney transplantation, and 1 patient with systemic lupus erythematosus) and 5 patients who were not immunocompromised. However, cryptococcal lymphadenitis in an HTLV-1 carrier was not reported. Based on the histopathological findings, granuloma formation, epithelioid cells, and necrotic portions were common in most cases, while gelatinous lesions and fibrosis were not observed. The number of fungal cocci per high power field in microscopy varied for each case. Tuberculous lymphadenitis also reportedly shows granuloma formation with a necrotic lesion.36 These results may suggest that epithelioid granuloma with necrotic tissue could indicate a diagnosis of cryptococcal lymphadenitis as well as tuberculous lymphadenitis.
Table 3. Previous reports about clinicopathological findings in cryptococcal lymphadenitis.
| Previous reports (References) |
Number of cases |
Onset age (years old) |
Immunological status | CD4+ cell count in PB (/mm3) |
Geratinous lesion |
Granuloma formation |
Epithelioid cells |
Fungal load | Necrosis |
|---|---|---|---|---|---|---|---|---|---|
| Kim et al8 | 1 | 42 | SLE | NA | NA | present | present | present | present |
| Gustafson et al4 | 1 | 24 | HIV carrier | 69 | NA | NA | NA | numerous | NA |
| Srinivasan et al9 | 15 | median age: 37 (range: 25-58) |
8 HIV carrier 5 non-immunocompromised 2 post kidney transplant |
NA | NA | 9 formed 6 not seen |
3 present 6 occasional 6 not seen |
11 numerous 4 scanty |
7 present 8 not seen |
| Dogbey et al3 | 1 | 38 | HIV carrier | 18 | NA | NA | NA | numerous | NA |
| Murakami et al15 | 1 | 26 | non-immnocompromised | normal (count unknown) |
NA | present | present | numerous | NA |
| Our study | 3 | 32, 43 85 |
2 HIV carrier 1 HTLV-1 carrier |
5.8, 79.9 3285 |
2 well-formed 1 formed |
2 formed 1 not seen |
2 not seen 1 occasional |
2 numerous 1 focal |
2 partially 1 not seen |
HIV: Human Immunodeficiency Virus, SLE: Systemic Lupus Erythematosus, HTLV-1: Human T-cell Leukemia Virus-1, PB: Peripheral Blood, FCM: Flow cytometry, NA: Not Available
In addition, the age of the HTLV-1 carrier with cryptococcosis was considerably higher than that of the HIV-1 carriers (Table 3). It is thought that immune aging might be one of the causes of cryptococcosis including cryptococcal lymphadenitis; the frequency of cryptococcosis may increase with increase in the long-term survival rate of HTLV-1 carriers.
In conclusion, based on the present patients, cryptococcal lymphadenitis might be associated with HIV infection and decreased CD4+ cell count, as previously suggested. In addition, it might be necessary to consider the possibility of the presence of cryptococcus lymphadenitis in HTLV-1 carriers and patients with a history of administration of immunosuppressive agents and/or anti-cancer agents, who have impaired normal CD4+ cells. On pathological images, epithelioid granuloma with necrotic tissue could suggest cryptococcal lymphadenitis.
ACKNOWLEDGEMENTS
The authors thank Mayumi Miura, Kazutaka Nakashima, Fumiko Arakawa, Kanoko Miyazaki, Yuki Morotomi, Chie Kuroki, and Kaoruko Nagatomo for their technical assistance.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflicts of interest.
REFERENCES
- 1.Mitchell TG, Perfect JR: Cryptococcosis in the era of AIDS--100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev 8: 515-548, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perfect JR: Cryptococcosis. Infect Dis Clin North Am 3: 77-102, 1989 [PubMed] [Google Scholar]
- 3.Bhuyan P, Pattnaik K, Kar A, Brahma RC, Mahapatra S: Cryptococcal lymphadenitis in HIV: a chance diagnosis by FNAC. Diagn Cytopathol 41: 456-458, 2013. 10.1002/dc.21805 [DOI] [PubMed] [Google Scholar]
- 4.Dogbey P, Golden M, Ngo N. Cryptococcal lymphadenitis: an unusual initial presentation of HIV infection. BMJ case-reports 2013, 2013 [DOI] [PMC free article] [PubMed]
- 5.Gustafson KS, Feldman L: Cryptococcal lymphadenitis diagnosed by fine-needle aspiration biopsy. Diagn Cytopathol 35: 103-104, 2007. 10.1002/dc.20596 [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Kim SD, Kim HR, Yoon CH, Lee SH, et al. : Intraabdominal cryptococcal lymphadenitis in a patient with systemic lupus erythematosus. J Korean Med Sci 20: 1059-1061, 2005. 10.3346/jkms.2005.20.6.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natukunda E, Musiime V, Ssali F, Kizito H, Kityo C, et al. : A Case of Cryptococcal Lymphadenitis in an HIV-Infected Child. AIDS Res Hum Retroviruses 27: 373-376, 2011. 10.1089/aid.2010.0167 [DOI] [PubMed] [Google Scholar]
- 8.Shravanakumar BR, Iyengar KR, Parasappa Y, Ramprakash R: Cryptococcal lymphadenitis diagnosed by FNAC in a HIV positive individual. J Postgrad Med 49: 370, 2003 [PubMed] [Google Scholar]
- 9.Srinivasan R, Gupta N, Shifa R, Malhotra P, Rajwanshi A, et al. : Cryptococcal lymphadenitis diagnosed by fine needle aspiration cytology: a review of 15 cases. Acta Cytol 54: 1-4, 2010. 10.1159/000324958 [DOI] [PubMed] [Google Scholar]
- 10.Baker RD, Haugen RK: Tissue changes and tissue diagnosis in cryptococcosis; a study of 26 cases. Am J Clin Pathol 25: 14-24, 1955. 10.1093/ajcp/25.1.14 [DOI] [PubMed] [Google Scholar]
- 11.Jagadha V, Andavolu RH, Huang CT: Granulomatous inflammation in the acquired immune deficiency syndrome. Am J Clin Pathol 84: 598-602, 1985. 10.1093/ajcp/84.5.598 [DOI] [PubMed] [Google Scholar]
- 12.Hill JO: CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J Exp Med 175: 1685-1695, 1992. 10.1084/jem.175.6.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pantanowitz L, Omar T, Sonnendecker H, Karstaedt AS: Bone marrow cryptococcal infection in the acquired immunodeficiency syndrome. J Infect 41: 92-94, 2000. 10.1053/jinf.2000.0667 [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto K, Miyoshi H, Yoshida N, Takizawa J, Sone H, et al. : Clinicopathological, Cytogenetic, and Prognostic Analysis of 131 Myeloid Sarcoma Patients. Am J Surg Pathol 40: 1473-1483, 2016. 10.1097/PAS.0000000000000727 [DOI] [PubMed] [Google Scholar]
- 15.Miyoshi H, Kiyasu J, Kato T, Yoshida N, Shimono J, et al. : PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood 128: 1374-1381, 2016. 10.1182/blood-2016-02-698936 [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi H, Sato K, Yoshida M, Kimura Y, Kiyasu J, et al. : CD5-positive follicular lymphoma characterized by CD25, MUM1, low frequency of t(14;18) and poor prognosis. Pathol Int 64: 95-103, 2014. 10.1111/pin.12145 [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto K, Miyoshi H, Yoshida N, Nakamura N, Ohshima K, et al. : MYC translocation and/or BCL 2 protein expression are associated with poor prognosis in diffuse large B-cell lymphoma. Cancer Sci 107: 853-861, 2016. 10.1111/cas.12942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamoto K, Miyoshi H, Yanagida E, Yoshida N, Kiyasu J, et al. : Comparison of clinicopathological characteristics between T-cell prolymphocytic leukemia and peripheral T-cell lymphoma, not other specified. Eur J Haematol 98: 459-466, 2017. 10.1111/ejh.12856 [DOI] [PubMed] [Google Scholar]
- 19.Marsh BJ: Infectious complications of human T cell leukemia/lymphoma virus type I infection. Clin Infect Dis 23: 138-145, 1996. 10.1093/clinids/23.1.138 [DOI] [PubMed] [Google Scholar]
- 20.Hanada S, Uematsu T, Iwahashi M, Nomura K, Utsunomiya A, et al. : The prevalence of human T-cell leukemia virus type I infection in patients with hematologic and nonhematologic diseases in an adult T-cell leukemia-endemic area of Japan. Cancer 64: 1290-1295, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Kashala O, Marlink R, Ilunga M, Diese M, Gormus B, et al. : Infection with human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotropic viruses among leprosy patients and contacts: correlation between HIV-1 cross-reactivity and antibodies to lipoarabinomannan. J Infect Dis 169: 296-304, 1994. 10.1093/infdis/169.2.296 [DOI] [PubMed] [Google Scholar]
- 22.Murai K, Tachibana N, Shioiri S, Shishime E, Okayama A, et al. : Suppression of delayed-type hypersensitivity to PPD and PHA in elderly HTLV-I carriers. J Acquir Immune Defic Syndr 3: 1006-1009, 1990 [PubMed] [Google Scholar]
- 23.Robinson RD, Lindo JF, Neva FA, Gam AA, Vogel P, et al. : Immunoepidemiologic studies of Strongyloides stercoralis and human T lymphotropic virus type I infections in Jamaica. J Infect Dis 169: 692-696, 1994. 10.1093/infdis/169.3.692 [DOI] [PubMed] [Google Scholar]
- 24.Sato Y, Shiroma Y: Concurrent infections with Strongyloides and T-cell leukemia virus and their possible effect on immune responses of host. Clin Immunol Immunopathol 52: 214-224, 1989. 10.1016/0090-1229(89)90173-6 [DOI] [PubMed] [Google Scholar]
- 25.Tachibana N, Okayama A, Ishizaki J, Yokota T, Shishime E, et al. Suppression of tuberculin skin reaction in healthy HTLV-I carriers from Japan. International journal of cancer Journal international du cancer 42(6):829-31, 1988 [DOI] [PubMed]
- 26.Verdier M, Denis F, Sangare A, Barin F, Gershy-Damet G, et al. : Prevalence of antibody to human T cell leukemia virus type 1 (HTLV-1) in populations of Ivory Coast, West. Afr J Infect Dis 160: 363-370, 1989. 10.1093/infdis/160.3.363 [DOI] [PubMed] [Google Scholar]
- 27.Verdier M, Denis F, Sangare A, Leonard G, Sassou-Guesseau E, et al. : Antibodies to human T lymphotropic virus type 1 in patients with leprosy in tropical areas. J Infect Dis 161: 1309-1310, 1990. 10.1093/infdis/161.6.1309 [DOI] [PubMed] [Google Scholar]
- 28.Michaels SH, Clark R, Kissinger P: Incidence and spectrum of AIDS-defining illnesses among persons treated with antiretroviral drugs. Clin Infect Dis 29: 468-469, 1999. 10.1086/520251 [DOI] [PubMed] [Google Scholar]
- 29.Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, et al. : Viral Activation Transfusion Study, Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med 135: 17-26, 2001. 10.7326/0003-4819-135-1-200107030-00005 [DOI] [PubMed] [Google Scholar]
- 30.Chang LW, Phipps WT, Kennedy GE, Rutherford GW: Antifungal interventions for the primary prevention of cryptococcal disease in adults with HIV. Cochrane Database Syst Rev 2005: CD004773, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Chetchotisakd P, Sungkanuparph S, Thinkhamrop B, Mootsikapun P, Boonyaprawit P: A multicentre, randomized, double-blind, placebo-controlled trial of primary cryptococcal meningitis prophylaxis in HIV-infected patients with severe immune deficiency. HIV Med 5: 140-143, 2004. 10.1111/j.1468-1293.2004.00201.x [DOI] [PubMed] [Google Scholar]
- 32.Jarvis JN, Casazza JP, Stone HH, Meintjes G, Lawn SD, et al. : The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. J Infect Dis 207: 1817-1828, 2013. 10.1093/infdis/jit099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy JW, Mosley RL: Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2). J Immunol 134: 577-584, 1985 [PubMed] [Google Scholar]
- 34.Ohshima K: Molecular Pathology of Adult T-Cell Leukemia/Lymphoma. Oncology 89(suppl 1): 7-15, 2015. 10.1159/000431058 [DOI] [PubMed] [Google Scholar]
- 35.Murakami Y, Oki M, Saka H, Kajikawa S, Murakami A, et al. : Disseminated cryptococcosis presenting as mediastinal and hilar lymphadenopathy in an immunocompetent patient. Respirol Case Rep 4: e00167, 2016. 10.1002/rcr2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontanilla JM, Barnes A, von Reyn CF: Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis 53: 555-562, 2011. 10.1093/cid/cir454 [DOI] [PubMed] [Google Scholar]