Abstract
Objectives
Recent technology advances have allowed for heart rhythm monitoring using single-lead ECG monitoring devices, which can be used for early diagnosis of atrial fibrillation (AF). We sought to investigate the AF detection rate using portable ECG devices compared with Holter monitoring.
Setting, participants and outcome measures
We searched the Medline, Embase and Scopus databases (conducted on 8 May 2017) using search terms related to AF screening and included studies with adults aged >18 years using portable ECG devices or Holter monitoring for AF detection. We excluded studies using implantable loop recorders and pacemakers. Using a random-effects model we calculated the overall AF detection rate. Meta-regression analysis was performed to explore potential sources for heterogeneity. Quality of reporting was assessed using the tool developed by Downs and Black.
Results
Portable ECG monitoring was used in 18 studies (n=117 436) and Holter monitoring was used in 36 studies (n=8498). The AF detection rate using portable ECG monitoring was 1.7% (95% CI 1.4 to 2.1), with significant heterogeneity between studies (p<0.001). There was a moderate linear relationship between total monitoring time and AF detection rate (r=0.65, p=0.003), and meta-regression identified total monitoring time (p=0.005) and body mass index (p=0.01) as potential contributors to heterogeneity. The detection rate (4.8%, 95% CI 3.6% to 6.0%) in eight studies (n=10 199), which performed multiple ECG recordings was comparable to that with 24 hours Holter (4.6%, 95% CI 3.5% to 5.7%). Intermittent recordings for 19 min total produced similar AF detection to 24 hours Holter monitoring.
Conclusion
Portable ECG devices may offer an efficient screening option for AF compared with 24 hours Holter monitoring.
PROSPERO registration number
CRD42017061021.
Strengths and limitations of this study.
First systematic review comparing single-lead ECG monitoring with 24 hours Holter monitoring for atrial fibrillation (AF) detection.
Comprehensive literature search and specific inclusion criteria allowing for large patient numbers.
Heterogeneity among individual studies with regard to patient population, AF definitions and monitoring time.
Poor reporting of CHA2DS2-VASC scores among individual studies.
Patient compliance unable to be accounted for in this meta-analysis.
Atrial fibrillation (AF) is a leading cause of stroke and heart failure worldwide, and is associated with increased all-cause mortality1 2 as well as substantial financial cost.3 4 The prevalence of AF increases with age, exceeding >15% for those aged 85 years and older.5 The epidemics of obesity, diabetes mellitus and metabolic syndrome have also been associated with the increasing prevalence of AF.6–8 Up to 20% of patients with stroke have underlying AF, and detection allows the initiation of anticoagulation, which is associated with a significant reduction in stroke recurrence.9
Early diagnosis of AF may have several benefits, including individualised lifestyle intervention10 and anticoagulation, and may be associated with a reduction in complications and healthcare costs. The importance of early diagnosis has been recognised in recent guidelines from the European Society of Cardiology, which recommended opportunistic screening using pulse palpation and 12-lead ECG.11 However, screening for AF is challenging for several reasons; many patients are asymptomatic or may have atypical symptoms. There are a variety of monitoring techniques available, all of which vary in diagnostic accuracy and sensitivity, and there is no accepted reference standard. Subclinical AF is associated with an increased risk of stroke, cardiovascular disease and all-cause mortality,12 although there is controversy surrounding the significance of brief paroxysms of AF and the potential benefit of anticoagulant therapy. Implantable devices are expensive, and not cost-effective for mass screening, and the use of external devices for long periods of monitoring require electrodes, which may be poorly tolerated by patients.
Recent advances in technology have allowed for the development of single-lead portable ECG monitoring devices. Multiple devices are available, all using multiple points of finger contact to create a single-lead ECG trace. The in-built memory of these devices allows for single or multiple time-point screening. Interpretation from a cardiologist or by automated algorithms has achieved high sensitivity and specificity for AF detection.13–15 Although they have not been incorporated into the latest AF guidelines, the accuracy, ease of use and potential cost-effectiveness of these devices may lead to them having an important role in AF screening. This paper describes a systematic review of the published literature to investigate the overall AF detection rate using portable ECG devices compared with traditional Holter monitoring.
Methods
Search strategy
We conducted our systematic review and meta-analysis using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline (PRISMA).16 We searched the Medline, Scopus and Embase databases using key terms including ‘atrial fibrillation/AF and screening/monitoring and electrocardiographic/Holter monitoring’, which were mapped to subject headings. We also searched the reference lists to identify other potential articles. The search was limited to adult human subjects aged >18 years and limited to the English language (see search strategy for Medline database in online supplementary material 1). The study was prospectively registered on the PROSPERO database on 22 April 2017 (CRD42017061021), and the search was conducted on 8 May 2017.
bmjopen-2018-024178supp001.pdf (338.7KB, pdf)
Study selection
Titles and abstracts of studies identified from the search were reviewed by two independent reviewers (SR and DDS). Studies which had a primary aim of AF detection in adult participants were included. We included all cohorts including community screening, those with risk factors and recent stroke. The screening methods included portable single-lead ECG devices or continuous (Holter) monitoring (up to 1 week). We included studies which used single-lead ECG devices for single episode screening or multiple intermittent screening periods. We included conference abstracts if demographic and outcome data were available. We excluded studies if participants were aged <18 years or if other forms of monitoring were used (pacemaker, implantable loop recorders, event recorders, monitoring patches and inpatient telemetry). We also excluded studies where AF detection was not the primary aim.
The primary outcome of interest was the detection rate of new AF using either single-lead intermittent or continuous monitoring. Our secondary objective was to determine the optimal time of intermittent monitoring, which produced equivalent AF detection to continuous monitoring.
Data collection
Full-text manuscripts of studies fitting the inclusion criteria were obtained. Quality of reporting and risk of bias was assessed using the tool developed by Downs and Black.17 A standardised data-extraction form was used by the reviewers, which included information about the patient demographics, comorbidities, screening strategy, patients with known AF and overall new AF detection rate. Where data were not reported, we attempted to contact the primary authors of the study. Any disagreements between the two reviewers were resolved by consensus or by consulting a third reviewer (THM).
Statistical analysis
The cumulative AF detection rate for continuous and intermittent monitoring and the 95% CI was calculated using a random-effects model. The results were displayed as a forest plot and heterogeneity among the studies was assessed using the I2 statistic. A subgroup analysis was performed by comparing the cumulative detection rate of single-lead ECG studies, which performed multiple timepoint recordings with 24 hours Holter monitoring studies. Linear regression analysis was used to determine the association between the total monitoring time and AF detection using single-lead ECG devices. This formula was used to determine the monitoring time using single-lead ECG devices to approximate the overall AF detection rate using 24 hours continuous monitoring. Univariate meta-regression analysis was performed to assess the influence of various clinical and screening factors with AF detection. Publication bias was assessed using a funnel plot and the Egger test. Statistical analysis was performed using Stata V.13 (StataCorp, College Station, Texas, USA) with two-tailed p values <0.05 used to denote statistical significance.
Patient and public involvement
Patients were not involved in this review.
Results
Study characteristics
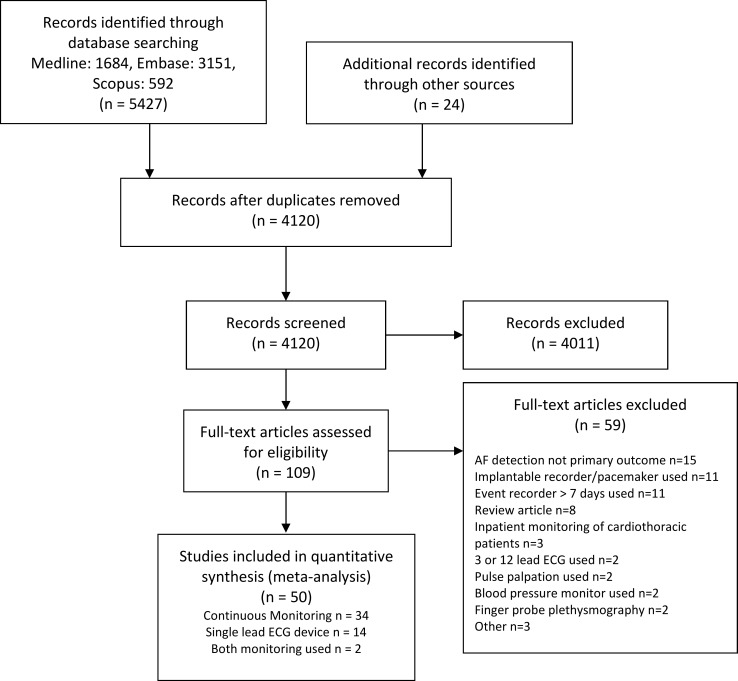
The PRISMA flow chart of our included studies is shown in figure 1 and the search strategy in online supplementary table 1. Our initial search strategy identified 5427 studies, with another 26 identified through other sources. After removing duplicate records, 4122 studies were left. After screening those using the inclusion/exclusion criteria, we identified 111 full-text studies for detailed review, which excluded 59 studies, leaving 52 full-text studies for inclusion in the meta-analysis (see online supplementary table 2 for excluded studies). Of the 52 studies included, 34 used continuous (Holter) monitoring (n=8154),18–51 16 studies (n=117 092) used single-lead portable ECG monitoring14 15 52–65 and 2 studies (n=344) used both continuous and intermittent single-lead monitoring for AF detection in a head-to-head comparison.66 67
Figure 1.
Overview of inclusion and exclusion of studies based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart.
The baseline characteristics of the individual studies is presented in table 1. There was a considerable range in age (54–76 years), and gender (male 29%–77%) between studies. As many studies chose healthy volunteers and other studies focused on patients poststroke or those with AF risk factors, there was significant variation in comorbidities such as diabetes, hypertension and obesity. Stroke risk determined by the CHADS or CHA2DS2-VASC score was reported in only 14/52 studies (27%). Of the 52 studies, 36 (69%) were conducted in Europe, 8 (15%) in Asia, 5 (10%) in North America and 3 (6%) in Australia. Nine studies (17%) were retrospective, the remainder all being prospective cohort or randomised controlled trials.
Table 1.
Summary of included trials investigating AF detection using single-lead ECG devices or Holter monitoring
| Study | n | Country | Type of patients used |
Device used |
Duration of recording (s) | Frequency of recording/ day |
Total monitoring (days) | Mean/ median age (years) |
Male (%) | BMI (kg/m2) | HTN (%) | DM (%) | IHD (%) | Previous diagnosis of AF (%) | HF (%) | Previous stroke (%) | Mean/ median CHADS/ CHA2DS2-VASC |
Definition of AF |
New AF (n) | New AF rate (%) |
| Lowres et al 52 | 1000 | Australia | Community pharmacy screening | Alive Cor | 60 | 1 | 0 | 76 | 44 | NR | 62 | 23 | 16 | 10.4 | 3 | 7 | 3.3 | Cardiologist interpretation |
15 | 1.5 |
| Svennberg et al 53 | 7173 | Sweden | Community screening (aged 75–76 years) | Zenicor | 30 | 2 | 14 | 75 | 46 | 25.9 | 50 | 11 | 9.2 | 9.2 | 3.4 | 9 | 3.4 | 30 s irregular rhythm without P waves or 2× episodes between 10 and 29 s | 218 | 3 |
| Proietti et al 54 | 65 747 | Belgium | Belgian Heart Week screening | Omron Heartscan HCG-801 |
30 | 1 | 0 | 58 | 41 | NR | 36 | 21 | 23 | 0.5 | 20 | 20 | 2 | Irregular R-R interval, no distinct P waves, variable atrial cycle length | 603 | 1.1 |
| Kaasenbrood et al 55 | 3269 | Holland | Influenza vaccination—opportunistic screening | MyDiagnostik | 60 | 1 | 0 | 64.1 | 49 | NR | NR | NR | NR | 2.6 | NR | NR | NR | Cardiologist interpretation×2 | 37 | 1.1 |
| Engdahl et al 56 | 848 | Sweden | Community screening (aged 75–76 years) in Halmstad, Sweden | Zenicor | 30 | 2 | 14 | 75 | 43 | NR | 53 | 11 | NR | 9.6 | 4 | 10 | 1.9 | 30 s duration of irregular rhythm or ≥2 episodes of 10 s or more | 40 | 4.7 |
| Hendrikx et al 57 | 928 | Sweden | GP practices |
Zenicor | 10 | 2 | 28 | 69.8 | 50 | NR | 90.3 | 31.6 | 19.8 | 0 | 3.7 | 8.6 | 2 | 10 s irregular rhythm without P waves | 35 | 3.8 |
| Hendrikx et al 67 | 95 | Sweden | Referred for presyncope/ palpitations |
Zenicor | 30 | 2 | 28 | 54.1 | 44 | NR | 28.4 | 1.1 | 8.4 | 0 | 0 | 6.3 | 1 | 30 s irregular rhythm without P waves | 9 | 9.5 |
| Chan et al 15 | 1013 | Hong Kong | Patients aged ≥65 years with HTN or diabetes | Alive Cor | 60 | 1 | 0 | 68.4 | 47 | NR | 90.4 | 36.6 | 16.2 | 2.2 | 4.4 | 10.5 | 3 | Cardiologist interpretation |
5 | 0.5 |
| Doliwa Sobocinski et al 66 |
249 | Sweden | Patients post-TIA/ stroke |
Zenicor | 10 | 2 | 30 | 72 | 57 | NR | 65 | 16 | 20 | 0 | 4 | 25 | 3 | Irregular rhythm of minimum 10 s without visible P waves | 15 | 6 |
| Doliwa Sobocinski P et al 14 |
606 | Sweden | Community event |
Zenicor | 10 | 1 | 0 | NR | 64 | NR | NR | NR | NR | NR | NR | NR | NR | Irregular rhythm without visible P waves | 6 | 1 |
| Ramkumar et al 60 | 204 | Australia | Community aged ≥65 years with one or more risk factor for HF | Remon RM-100 | 60 | 5 | 7 | 70.1 | 51 | 29.1 | 72.1 | 56.4 | 5.9 | 0 | 0 | NR | 3 | 30 s duration of irregular rhythm with absent P waves | 20 | 9.8 |
| Hendrikx et al 58 | 201 | Sweden | Patients referred to respiratory clinics with suspicion of obstructive sleep apnoea | Zenicor | 30 | 2 | 14 | 56 | 69 | 30 | 51 | 10 | 9.2 | 0 | 4.6 | 3.1 | NR | Irregular supraventricular extra systoles in series for 30 s | 13 | 6.5 |
| Claes et al 61 | 10 758 | Belgium | Community heart rhythm screening programme through medical centres | Omron HeartScan HCG-801 |
30 | 1 | 0 | 59 | 38 | NR | 30.6 | 8.6 | 12.2 | 7.2 | 7.2 | 5.4 | 1 | Irregular RR intervals, absence of P waves and variable atrial cycle length (when visible) | 167 | 1.6 |
| Samol et al 62 | 132 | Germany | Large proportion poststroke/TIA. Also recruited from diabetes, HTN and dyslipidemia clinics | Omron HeartScan HCG-801 |
30 | 1 | 0 | 64 | 58 | NR | 67 | 27 | NR | 0 | 3 | 49 | NR | Cardiologist Interpretation×2 | 7 | 5.3 |
| Battipaglia et al 63 | 855 | UK | Community shopping centre screening | MyDiagnostik | 15 | 1 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 7 | 0.8 |
| Chan and Choy59 | 13 122 | Hong Kong | Nationwide community screening programme | Alive Cor | 30 | 1 | 0 | 64.7 | 29 | 23.7 | 38.2 | 14.8 | 2.2 | 0 | 0.7 | 2.8 | NR | Software algorithm definition with minimum of 30 s | 101 | 0.8 |
| Chan et al 65 | 10 735 | Hong Kong | Nationwide community screening programme | Alive Cor | 30 | 1 | 0 | NR | NR | NR | NR | NR | NR | 1.2 | NR | NR | NR | Cardiologist interpretation (≥30 s) |
74 | 0.7 |
| Halcox et al 64 | 501 | UK | Community based with individuals aged >65 years with CHA2DS2-VASC score≥2 |
Alive Cor | 30 | 2× per week | 365 | 72.6 | 48 | NR | 54 | 26 | 14 | 0 | 1.0 | 7.0 | 3.0 | 30 s duration of an irregular rhythm without P waves | 19 | 3.8 |
| Gladstone et al 18 | 277 | Canada | Patients admitted with cryptogenic stroke | Holter | Continuous | Continuous | 1 | 73.2 | 56 | NR | 67 | 19.3 | 14.7 | 0 | 7 | 12.6 | NR | 30 s or longer duration of irregular rhythm | 9 | 3.2 |
| Barthélémy et al 19 | 60 | France | Consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 64.4 | 55 | NR | 50 | 17 | NR | 0 | NR | 27 | NR | Fibrillatory waves associated with irregular ventricular response ratio at least 30 s duration | 8 | 13.3 |
| Jabaudon et al 20 | 149 | Switzerland | Consecutive patients admitted with stroke/TIA |
Holter | Continuous | Continuous | 1 | 66.9 | 68 | NR | 58 | 16.7 | 16.8 | 4.7 | NR | 16.8 | NR | NR | 7 | 4.7 |
| Koudstaal et al 21 | 100 | Holland | Retrospective study of 100 patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 60.9 | 74 | NR | NR | NR | 41 | NR | NR | NR | NR | NR | 5 | 5 |
| Hornig et al 22 | 268 | Germany | Consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 59.1 | 61 | NR | 43.7 | 34 | NR | NR | 14.9 | 45 | NR | NR | 10 | 3.3 |
| Rizos et al 23 | 496 | Germany | Patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 69 | 62 | NR | 78.8 | 24.6 | NR | NR | NR | 22.2 | 3 | Cardiologist interpretation (≥30 s) |
14 | 2.8 |
| Schuchert et al 24 | 82 | Germany | Consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 3 | 59.7 | 57 | NR | 36.5 | NR | 17.1 | NR | NR | NR | NR | Small irregular baseline undulations of variable amplitudes and morphology at a rate >350/min with an irregular ventiruclar response for at least 1 min | 5 | 6 |
| Schaer et al 25 | 241 | Switzerland | Consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 68.7 | 59 | NR | 76 | 25 | 41 | 7 | NR | 4.6 | NR | NR | 0 | 0 |
| Schaer et al 26 | 425 | Switzerland | Retrospective review of patients poststroke/TIA with Holter monitoring | Holter | Continuous | Continuous | 1 | 67.4 | 61 | NR | NR | NR | NR | NR | NR | 1.2 | NR | Self-terminating sequence of >30 s of irregular RR intervals and the presence of fibrillatory P waves | 9 | 2.1 |
| Shafqat et al 27 | 465 | Pakistan | Retrospective review of consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 66.8 | 56 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 5 | 2.4 |
| Lazzaro et al 28 | 133 | USA | Consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 63.1 | 50 | NR | 70 | 29.3 | 18.8 | 0 | NR | 2.3 | NR | Supraventricular tachyarrhythmia characterised by uncoordinated atrial activation with fibrillatory waves varying in amplitude, shape and timing, replacing consistent P waves and with a duration >30 s | 8 | 6 |
| Grond et al 29 | 1135 | Germany | Patients admitted in seven German centres with stroke/TIA | Holter | Continuous | Continuous | 3 | 67 | 55 | 27.4 | 20.4 | 7.3 | 0 | 5.8 | 17.4 | NR | ≥1 period of >30 s duration of an absolute arrhythmia without detectable P waves and without a pattern more consistent with an alternate diagnosis | 49 | 4.3 | |
| Stahrenberg et al 30 | 224 | Germany | Consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 7 | 68 | 58 | 27.6 | 72.9 | 22.3 | 14.8 | 0 | 5.2 | 16.2 | NR | 2x Cardiologist interpretation of software algorithm detection of events | 28 | 12.5 |
| Ritter et al 31 | 60 | Germany | Patients admitted with cryptogenic stroke | Holter | Continuous | Continuous | 7 | 61.8 | 57 | NR | 70 | 11.7 | 13.3 | NR | 0 | NR | 4 | Cardiologist interpretation (>30 s) |
1 | 1.7 |
| Higgins et al 32 | 50 | Scotland | Patients admitted with stroke/TIA | Holter | Continuous | Continuous | 7 | 67.1 | 48 | NR | 56 | 8 | 16 | 0 | NR | NR | NR | Cardiologist interpretation (>30 s) |
4 | 8 |
| Hendrikx et al 67 | 95 | Sweden | Patients investigated for palpitations and presyncope | Holter | Continuous | Continuous | 1 | 54.1 | 42 | NR | 28.4 | 1.1 | 8.4 | 0 | 0 | 6.3 | 1 | 30 s irregular rhythm without P waves |
2 | 2.1 |
| Thakkar and Bagarhatta33 | 52 | India | Consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 59.5 | 77 | NR | 51.9 | 23.1 | 15.4 | 0 | 1.7 | 7.7 | NR | 30 s irregular rhythm without P waves |
3 | 5.8 |
| Wachter et al 34 | 198 | Germany | Consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 73.2 | 62 | NR | 80.7 | 26.4 | 9.1 | 0 | 4.6 | 21.7 | 4.8 | >30 s rhythm with irregular RR intervals and the presence of fibrillatory P waves | 9 | 5 |
| Gumbinger et al 35 | 192 | Germany | Patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 2 | 1 |
| Alhadramy et al 36 | 426 | Canada | Retrospective review of patients poststroke/TIA with Holter monitoring | Holter | Continuous | Continuous | 1 | 64.9 | 48 | NR | 58.2 | 14.1 | 14.1 | 0 | 1.6 | 6.3 | NR | Irregular ventricular response in the absence of p waves or with fibrillatory waves | 11 | 2.5 |
| Doliwa Sobocinski et al 66 | 249 | Sweden | Consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 72 | 57 | NR | 65 | 16 | 20 | 0 | 4 | 25 | 3 | Irregular rhythm of minimum 10 s without visible P waves | 5 | 2 |
| Dangayach et al 37 | 51 | USA | Retrospective audit of patients admitted with cryptogenic stroke | Holter | Continuous | Continuous | 2 | 58.2 | 43 | NR | 35.3 | 16 | 15.7 | 7.4 | NR | NR | NR | NR | 15 | 29.4 |
| Gunalp et al 38 | 26 | Turkey | Patients admitted with ischaemic stroke | Holter | Continuous | Continuous | 1 | 66 | 69 | NR | 61 | 26 | 31 | NR | NR | NR | NR | NR | 11 | 42.3 |
| Fonseca et al 39 | 80 | Portugal | Patients admitted with cryptogenic stroke | Holter | Continuous | Continuous | 1 | 69.3 | 53 | NR | 71.3 | 28.8 | 11.3 | NR | NR | 22.5 | NR | NR | 17 | 21 |
| Manina et al 40 | 114 | Italy | Patients admitted with cryptogenic stroke | Holter | Continuous | Continuous | 4 | 63.1 | NR | NR | 52.6 | 9.6 | NR | NR | NR | NR | NR | Irregular ventricular response in the absence of P waves or with fibrillatory waves | 29 | 25.4 |
| Tagawa et al 41 | 308 | Japan | Consecutive patients admitted with ischaemic stroke | Holter | Continuous | Continuous | 1 | 72.6 | 60 | NR | 70.1 | 25.3 | NR | 20.4 | NR | NR | NR | Small irregular baseline undulations of variable amplitude and morphology at a rate of 300–350/min associated with irregular ventricular response | 26 | 8.4 |
| Shibazaki et al 42 | 536 | Japan | Consecutive patients admitted with ischaemic stroke | Holter | Continuous | Continuous | 1 | 72.4 | 64 | NR | 65.9 | 25.7 | 9.8 | NR | 0.3 | NR | NR | NR | 12 | 2.2 |
| Vandebroucke and Thijs43 | 136 | Belgium | Retrospective audit of patients admitted with ischaemic stroke | Holter | Continuous | Continuous | 1 | 68 | 52 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 7 | 5.1 |
| Yodogawa et al 44 | 68 | Japan | Consecutive patients admitted with ischaemic stroke | Holter | Continuous | Continuous | 1 | 69.9 | 54 | NR | 66.2 | 14.7 | NR | NR | NR | NR | NR | Irregular and uncoordinated atrial electrical activity on surface ECG lasting >30 s | 17 | 25 |
| Atmuri et al 45 | 140 | Australia | Retrospective audit of patients admitted with ischaemic stroke/TIA | Holter | Continuous | Continuous | 1 | NR | NR | NR | 65 | 20 | 37.1 | 18.6 | NR | NR | NR | NR | 12 | 8.6 |
| Salvatori et al 46 | 274 | Italy | Cohort study of patients aged ≥65 years with HTN in multiple GP clinics | Holter | Continuous | Continuous | 2 | 70 | 54 | NR | 100 | 15 | 9 | 7 | 4 | 2.2 | NR | Cardiologist interpretation |
4 | 1.5 |
| Beaulieu-Boire et al 47 | 284 | Canada | Consecutive patients admitted with stroke/TIA | Holter | Continuous | Continuous | 1 | 70.6 | 52 | NR | 68.7 | 26.7 | 27.4 | NR | 2.2 | 22.3 | NR | Cardiologist interpretation |
18 | 6.3 |
| Dogan et al 48 | 400 | Turkey | Retrospective review of patients admitted poststroke | Holter | Continuous | Continuous | 1 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 40 | 10 |
| Douen et al 49 | 126 | Canada | Retrospective review of patients admitted poststroke | Holter | Continuous | Continuous | 1 | NR | NR | NR | NR | NR | NR | 7 | NR | NR | NR | NR | 9 | 7.1 |
| Suissa et al 50 | 354 | France | Consecutive patients admitted with ischaemic stroke | Holter | Continuous | Continuous | 1 | 62.4 | 57 | NR | 51.1 | 18.6 | NR | 0 | NR | NR | NR | Cardiologist interpretation |
2 | 0.6 |
| Wohlfahrt et al 51 | 224 | Germany | Patients admitted with ischaemic stroke | Holter | Continuous | Continuous | 7 | 68.5 | 59 | NR | 73.2 | 22.3 | 15.2 | NR | 5.4 | 24.1 | NR | >30 s irregular rhythm |
29 | 12.9 |
AF, atrial fibrillation; BMI, body mass index; DM, diabetes mellitus; GP, general practitioner; HF, heart failure; HTN, hypertension; IHD, ischaemic heart disease.
Of the 18 studies using single-lead ECG devices, 10 studies (56%) used a single 10–60 s recording for AF detection while 8 studies (44%) used multiple readings over a 1-week to 52-week period. There were five portable ECG devices used (table 1). Sixteen studies (89%) used healthy participants with risk factors.14 15 52–61 63–65 67 Two studies assessed patients following stroke or transient ischaemic attack (TIA).62 66
Of the 36 studies using continuous (Holter) monitoring, 27 studies (75%) used 24 hours continuous monitoring,18–23 25–28 33–36 38 39 41–45 47–50 66 674 studies (11%) used 1-week monitoring,30–32 51 2 studies (6%) used 48 hours monitoring,37 46 2 studies (6%) used 72 hours monitoring24 29 and 1 study (3%) used 96 hours monitoring.40
Overall AF detection
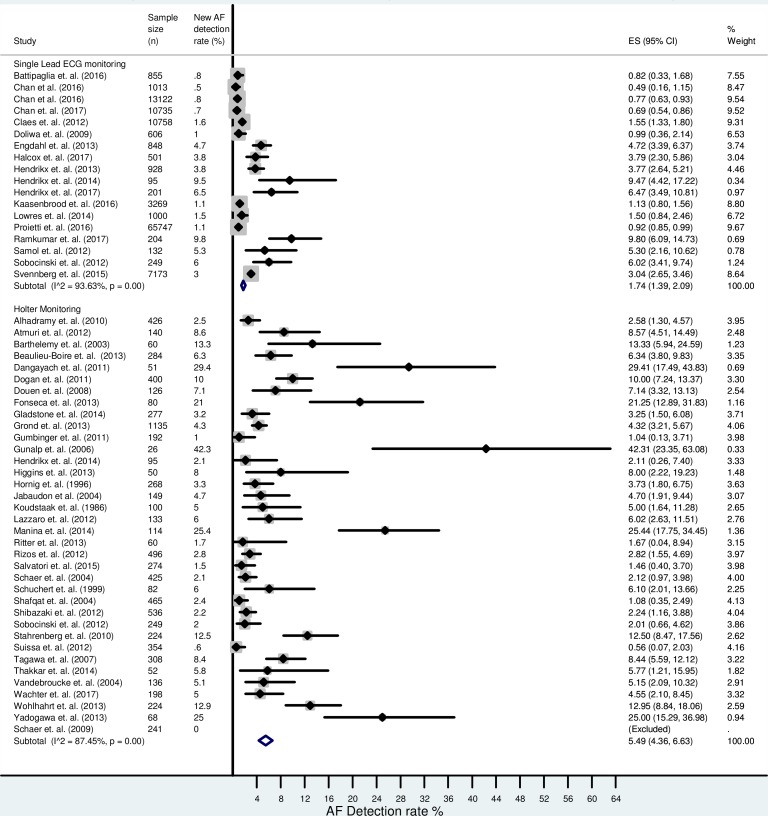
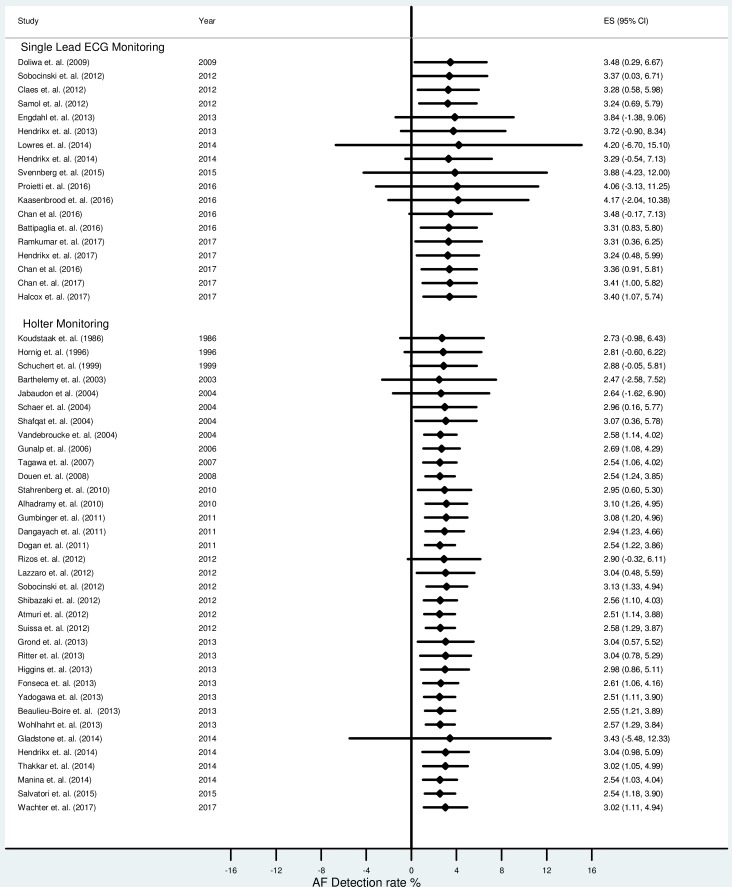
The combined AF detection rate using single-lead ECG monitoring (n=117 436 from 18 studies) was 1.7% (95% CI 1.4% to 2.1%). The cumulative AF detection rate using continuous (Holter) monitoring (n=8498 from 36 studies) was 5.5% (95% CI 4.4% to 6.6%). There was significant heterogeneity between studies (I2=94% for single-lead ECG monitoring, 87% for Holter monitoring). The overall new AF detection rate is presented in figure 2.
Figure 2.
Forest plot showing the overall atrial fibrillation (AF) detection rate between single-lead ECG devices and Holter monitoring.
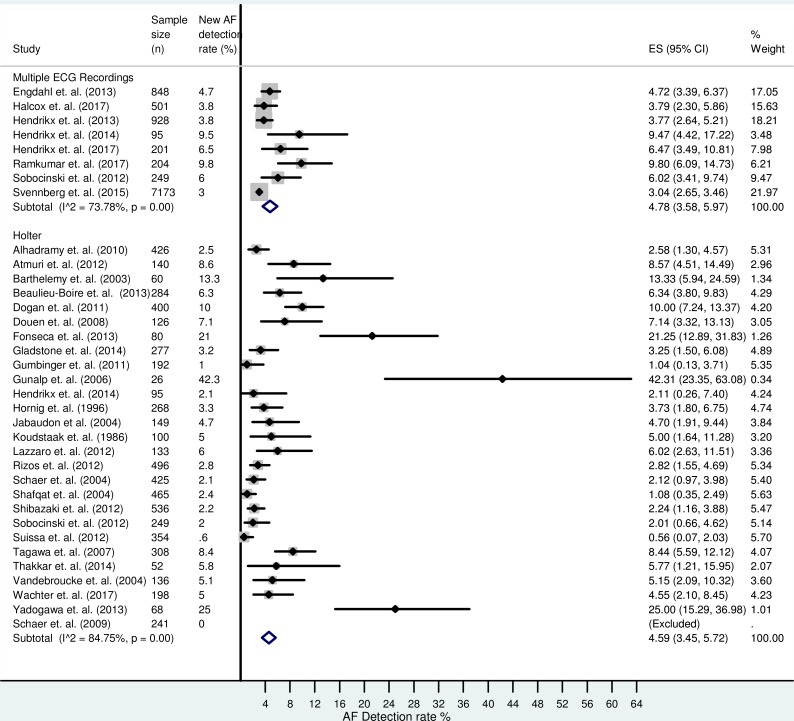
Comparison of multiple intermittent monitoring with 24 hours Holter
There was significant variation in the monitoring time using both single-lead and Holter monitoring, which contributed to the difference in the cumulative detection rate seen in figure 2. Figure 3 compares the detection rate of multiple intermittent single-lead recordings with 24 hours continuous monitoring, which is used routinely in clinical practice. There were eight studies (n=10 199, mean weighted age 68.8±8.4 years from six studies, 47% male from eight studies) that performed multiple intermittent single-lead ECG recordings and 27 studies (n=6284, mean weighted age 67.8±5.1 years from 23 studies, 58% male from 23 studies) that used 24 hours Holter monitoring. From the data available, the multiple intermittent ECG group had a lower AF risk to the 24 hours Holter group (hypertension 55% (n=8 studies) vs 65% (n=20 studies); diabetes mellitus 15% (n=8 studies) vs 22% (n=20 studies); heart failure 3.3% (n=8 studies) vs 3.9% (n=11 studies); ischaemic heart disease 11% (n=6 studies) vs 19% (n=15 studies) and previous stroke/TIA 9% (n=7 studies) vs 16% (n=15 studies)), respectively. The combined AF detection rate was 4.8% (95% CI 3.6% to 6.0%) using multiple intermittent ECG recordings. The cumulative AF detection rate using 24 hours Holter monitoring was 4.6% (95% CI 3.5% to 5.7%).
Figure 3.
Forest plot comparing the atrial fibrillation (AF) detection rate between 24 hours Holter monitoring and performing multiple intermittent single-lead ECG recordings.
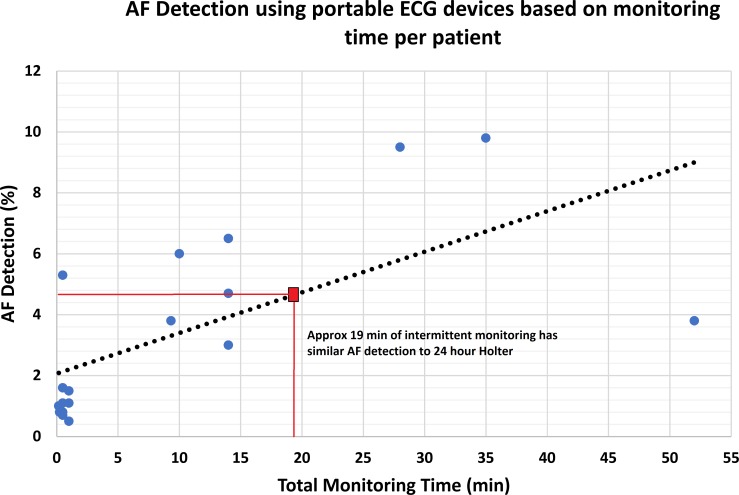
Association between monitoring time and AF detection
Using single-lead ECG devices, we found a moderate linear relationship between the total monitoring time and AF detection rate (β=0.13, R2=0.42). Using this formula, we noted that approximately 19 min of total intermittent monitoring produced similar AF detection to 24 hours continuous monitoring (figure 4). The study by Halcox et al was an outlier, with a much lower AF detection rate than other studies (3.8% from 52 min of total monitoring) and this reduced the linear correlation between total monitoring time and AF detection rate.64 Exclusion of these data led to a stronger linear relationship (β=0.26, R2=0.80) and a much lower total intermittent monitoring time required (12 min) to produce a similar AF detection rate to 24 hours Holter monitoring.
Figure 4.
Graph showing the linear relationship between total monitoring time and atrial fibrillation (AF) detection rate in single-lead ECG devices.
Meta-regression
Sources of heterogeneity in the 18 studies using single-lead ECG monitoring were investigated using meta-regression (table 2). Monitoring time per participant (β=0.11, 95% CI 0.04 to 0.18, p=0.005) and body mass index (β=1.1, 95% CI 0.58 to 1.5, p=0.01) were associated with AF detection.
Table 2.
Meta-regression analysis for atrial fibrillation (AF) detection (single-lead ECG studies)
| Variable | Number of studies | β (95% CI) | P values |
| Age (years) | 15 | 0.00 (−0.22 to 0.24) | 0.95 |
| Monitoring time per participant (min) | 18 | 0.11 (0.04 to 0.18) | 0.005 |
| Body mass index (kg/m2) | 4 | 1.1 (0.58 to 1.5) | 0.01 |
| CHADS score (%) | 11 | −0.13 (−2.6 to 2.4) | 0.91 |
| Hypertension (%) | 14 | 0.01 (−0.08 to 0.10) | 0.75 |
| Previous diagnosis of AF (%) | 16 | −0.13 (−0.50 to 0.24) | 0.46 |
| Ischaemic heart disease (%) | 12 | −0.10 (−0.42 to 0.21) | 0.48 |
| Previous stroke (%) | 13 | 0.06 (−0.09 to 0.19) | 0.45 |
| Male gender | 16 | 0.10 (−0.04 to 0.24) | 0.16 |
Sensitivity analysis
A number of outlier studies were observed in the meta-analysis that could influence the cumulative AF detection rate.37–40 44 Removal of these outlier studies resulted in a reduction in the overall AF detection rate in all Holter studies (table 3) and for 24 hours Holter studies (table 4). When these outlier studies were removed, the overall AF detection rate for 24 hours Holter was 3.86% (95% CI 2.88% to 4.83%), much lower than the detection rate by multiple intermittent ECG recordings using portable single lead devices (4.78%, 95% CI 3.58% to 5.97%). A cumulative meta-analysis (figure 5) did not show any significant variation in the AF detection rate over time using either Holter or single-lead ECG monitoring.
Table 3.
Outlier studies omitted (all Holter studies) to assess the change to the overall atrial fibrillation (AF) detection rate
Table 4.
Outlier studies omitted (24 hours Holter) to assess the change to the overall atrial fibrillation (AF) detection rate
Figure 5.
Cumulative meta-analysis showing minimal variation in atrial fibrillation (AF) detection over time using Holter and single-lead ECG devices.
Publication bias
Publication bias was explored using a funnel plot of all included studies (see online supplementary figure 1). There was significant publication bias in both single-lead ECG device and Holter monitoring studies (Egger test, p=0.003 and p<0.001 respectively).
Quality of studies
A summary of the quality analysis (see online supplementary table 3) showed that overall quality of reporting was moderate. All studies described the primary objective of the trial and included a summary of the main findings. Detailed comorbidities of the study participants were only adequately reported in 28/52 (54%), and limitations were discussed in 35/52 (67%) of studies. Most had a very selective patient population, 31/52 (60%) were poststroke/TIA cohorts.
Discussion
Our study is the only systematic review that we are aware of that has studied the overall AF detection rate of single-lead portable ECG devices. The results of our systematic review suggest a linear relationship between monitoring time per patient and AF detection rate. Single timepoint screening has an approximate 1% AF detection rate, which can be increased to around 5% when multiple recordings are performed. We noted that approximately 19 min of intermittent monitoring produced similar detection rates to conventional 24 hours continuous Holter monitoring.
Early diagnosis of AF
AF creates a significant burden on both patients as well as the healthcare system. AF will continue to rise in incidence and the costs to the healthcare system will continue to increase, due to ageing, sedentariness and the prevalence of obesity and the metabolic syndrome.3 68 Early diagnosis offers the possibility for early initiation of treatment, which may reduce the occurrence of the complications and may lead to reduced hospital admissions and associated healthcare costs. Early treatment for AF can be achieved in different ways. Patients with subclinical AF have an increased risk of stroke and cardiovascular events, like those with established AF.12 69 Anticoagulation may help reduce the incidence of stroke in this cohort.
The close relationship between metabolic syndrome and AF has encouraged research into the benefits of lifestyle intervention. Aggressive lifestyle intervention in patients with AF undergoing catheter ablation has been reported to lead to a reduction in symptom burden, improved quality of life and the need for repeat ablation procedures.10 It remains to be tested whether initiation of lifestyle intervention and aggressive risk factor modification following the early diagnosis of AF may be associated with positive LA remodelling and reduction of disease progression. Such a process may lead to additional health benefits, including reduction in cardiovascular risk and improvement in exercise capacity.
AF screening and feasibility
AF is a leading cause of stroke and heart failure in the community. As well as an association with increased all-cause mortality, it is associated with reduced quality of life. The availability of preventive therapies, including anticoagulation, has led to increasing recognition of the importance of AF screening for early diagnosis. However, AF screening shares the limitations of screening with other diagnostic tests. The screening tool must have high sensitivity, and needs to be inexpensive and cost-effective. We also need to minimise and have a method of addressing false positives. Current guidelines recommend opportunistic screening using pulse palpation and 12-lead ECG.11 In a previous systematic review, this was associated with a new AF detection rate of approximately 1%.5 Pulse palpation may be non-specific in patients with other irregular rhythms such as ventricular ectopy, and 12-lead ECG is only able to capture a single timepoint for screening. There are multiple other methods for AF detection. Continuous Holter monitoring is probably the most commonly used in clinical practice, especially in stroke cohorts. It has the potential advantage of assessing heart rhythm throughout the day and may be useful in detecting nocturnal subclinical AF. However, the disadvantages include the cost of Holter monitoring (especially for mass screening), the inconvenience of leads and electrodes (which may affect compliance) and typical limitation to 1–2 days of capture (as extended periods are more cumbersome and less cost-effective). Other event recorders are again expensive and limited to symptomatic patients. Extended period monitoring using implantable devices have shown promise in the cryptogenic stroke population (where many have been diagnosed with paroxysmal AF),70 but they are invasive and not feasible for mass screening.
Portable single-lead ECG devices permit multiple 30–60 s recordings to be captured, and downloaded to a computer. These devices have several potential advantages over Holter monitoring. They are leadless and require finger contact (and are hence easy to use and acceptable to patients). They have a high degree of sensitivity for identifying AF.71–73 Most interface with a web-based cloud system where ECG rhythms can be wirelessly transferred to clinicians, allowing rapid analysis and diagnosis. The development of automated algorithms to detect AF is helpful for mass screening. In two small studies they have demonstrated superior AF detection compared with 24 hours Holter monitoring.66 67 Although screening using these portable devices are currently not in the latest AF guidelines, they may offer a feasible option for mass screening. Screening using these devices has been demonstrated to be cost-effective.74 75
We noted a moderate linear association between monitoring time and AF detection rate. Single timepoint screening for 30–60 s achieved an overall detection rate of approximately 1%. This is no better than what has been reported using pulse palpation or 12-lead ECG, hence does not add any incremental benefit in screening programmes.5 Multiple intermittent recordings improve AF detection; we found that at least 19 min of total monitoring should be performed to achieve detection rates similar to 24 Holter monitoring.
The linear relationship between monitoring time and AF detection rate (R2=0.80) and the reproduction of AF detection rates of 24 hours Holter monitoring with only 12 min of intermittent monitoring was possible in our study only after exclusion of an outlier.64 Despite the inclusion of elderly participants with at least one risk factor for AF, the use of a validated single-lead ECG device and a prolonged monitoring period, that study had a lower AF detection rate (3.8%) than the remaining studies, even using a shorter monitoring period.53 56 57 Relatively low rates of adherence (only approximately 25% completed 2×30 s ECG recordings every week for the full year of monitoring) may be a potential explanation for the lower AF detection rate noted.64
Limitations
There are several challenges inherent in this meta-analysis of studies investigating AF detection. The most important is the target screening population. Most studies did not report the CHADS or CHA2DS2-VASC score, a history of previous stroke or other comorbidities. Consequently, it was difficult to ascertain if the risk profiles of patients in these studies were equivalent. Most Holter monitoring studies were performed in the stroke population—which is likely a population with higher AF risk than many studies using portable ECG devices, which recruited mainly healthy participants or those with AF risk factors from the community. The significant heterogeneity among both Holter and portable ECG device studies make it difficult to perform direct comparisons between both groups. The type/duration of monitoring and type of device used will also influence the overall AF detection rate and varied significantly between studies. There are several possible confounders which may not have been taken into account. The validity of the linear regression analysis comparing detection time and rate may be limited due to the significant differences in study population, study design and AF definitions. However, despite these limitations, the analysis may provide some important inferences into AF screening. Multiple intermittent ECG recordings achieved a similar AF detection rate to 24 hours Holter monitoring. This may suggest that in a similar cohort of patients with the same comorbidities, single-lead intermittent monitoring may be superior for AF detection.
Compared with 24 hours continuous monitoring, single-lead portable ECG monitoring is more patient dependent. Good patient compliance is essential to obtain multiple readings across different timepoints which improves sensitivity. The analysis performed does not take into account patient compliance as this is difficult to assess and poorly reported across the individual studies. Most single-lead device manufacturers have proprietary automated AF detection algorithms, which were used for diagnosis. Not all of these algorithms have had rigorous testing and comparison to a reference standard. It is also difficult to distinguish AF from other supraventricular tachycardias using single-lead ECG devices as the P wave is often not readily discernible. The use of different automated algorithms makes AF definitions non-standardised and can potentially create issues with both overdiagnosis and underdiagnosis.
There are other limitations in this analysis. The efficacy of intermittent monitoring is critically dependent on AF burden and density. All studies varied in their monitoring period and strategy. The linear regression model used was able to determine a total intermittent monitoring time, which produced similar AF detection rates to 24 hours continuous monitoring. However, it is difficult to translate the total monitoring time into an effective monitoring strategy. For example, we are unable to determine from our analysis if 12×60 s recordings over 12 consecutive days is different to 2×60 s recordings daily for six consecutive days. The definitions of AF also vary between studies. Many are based on individual physician interpretation and criteria for diagnosis were not explicitly specified. The duration of AF varied from 10 to 30 s between studies, although a cut-off of 30 s was the most widely adopted practice.
Conclusion
Single-lead portable ECG devices may offer an efficient screening option for AF compared with 24 hours Holter monitoring. Total monitoring time is related to AF detection and a total of 19 min may achieve a similar detection rate to 24 hours Holter monitoring.
Supplementary Material
Footnotes
Contributors: SR performed the literature search and analysis of individual studies. Involved in the statistical analysis, manuscript preparation and editing. THM is guarantor. Developed project idea/rationale. Involved in data analysis and manuscript preparation and editing. NN was involved in data and statistical analysis as well as manuscript preparation and editing. DDS performed the literature search and analysis of individual studies. Involved in the manuscript preparation and editing. DJP was involved in analysis of individual studies and statistical analysis. Involved in manuscript preparation and editing. JMK was involved in the project outline, data analysis, manuscript preparation and editing.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: The sponsors had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data and in the preparation, review or approval of the manuscript.
Competing interests: SR receives equipment and software support from Semacare, a manufacturer of handheld ECG devices. Receives research scholarships from the Heart Foundation and Avant. THM receives equipment and software support from Semacare.
Patient consent: Not requried.
Ethics approval: Tasmanian Health and Medical Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no remaining unpublished data.
References
- 1. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the manitoba follow-up study. Am J Med 1995;98:476–84. 10.1016/S0002-9343(99)80348-9 [DOI] [PubMed] [Google Scholar]
- 2. Vidaillet H, Granada JF, Chyou P, et al. A population-based study of mortality among patients with atrial fibrillation or flutter. Am J Med 2002;113:365–70. 10.1016/S0002-9343(02)01253-6 [DOI] [PubMed] [Google Scholar]
- 3. Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313–20. 10.1161/CIRCOUTCOMES.110.958165 [DOI] [PubMed] [Google Scholar]
- 4. Leyden JM, Kleinig TJ, Newbury J, et al. Adelaide stroke incidence study: declining stroke rates but many preventable cardioembolic strokes. Stroke 2013;44:1226–31. 10.1161/STROKEAHA.113.675140 [DOI] [PubMed] [Google Scholar]
- 5. Lowres N, Neubeck L, Redfern J, et al. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost 2013;110:213–22. 10.1160/TH13-02-0165 [DOI] [PubMed] [Google Scholar]
- 6. Watanabe H, Tanabe N, Watanabe T, et al. Metabolic syndrome and risk of development of atrial fibrillation. Circulation 2008;117:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ball J, Thompson DR, Ski CF, et al. Estimating the current and future prevalence of atrial fibrillation in the Australian adult population. Med J Aust 2015;202:32–5. 10.5694/mja14.00238 [DOI] [PubMed] [Google Scholar]
- 8. Dagres N, Anastasiou-Nana M. Atrial Fibrillation and Obesity. J Am Coll Cardiol 2010;55:2328–9. 10.1016/j.jacc.2010.01.045 [DOI] [PubMed] [Google Scholar]
- 9. Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2015;14:377–87. 10.1016/S1474-4422(15)70027-X [DOI] [PubMed] [Google Scholar]
- 10. Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–31. 10.1016/j.jacc.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 11. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 12. Martinez C, Katholing A, Freedman SB. Adverse prognosis of incidentally detected ambulatory atrial fibrillation. A cohort study. Thromb Haemost 2014;112:276–86. 10.1160/TH4-04-0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lau J, Lowres N, Neubeck L, et al. Performance of an automated iPhone ECG algorithm to diagnose atrial fibrillation in a community AF screening program (SEARCH-AF). Heart, Lung and Circulation 2013;22:S205 10.1016/j.hlc.2013.05.488 [DOI] [Google Scholar]
- 14. Doliwa PS, Frykman V, Rosenqvist M. Short-term ECG for out of hospital detection of silent atrial fibrillation episodes. Scand Cardiovasc J 2009;43:163–8. 10.1080/14017430802593435 [DOI] [PubMed] [Google Scholar]
- 15. Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016;5:e003428 10.1161/JAHA.116.003428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. 10.1056/NEJMoa1311376 [DOI] [PubMed] [Google Scholar]
- 19. Barthélémy JC, Féasson-Gérard S, Garnier P, et al. Automatic cardiac event recorders reveal paroxysmal atrial fibrillation after unexplained strokes or transient ischemic attacks. Ann Noninvasive Electrocardiol 2003;8:194–9. 10.1046/j.1542-474X.2003.08305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jabaudon D, Sztajzel J, Sievert K, et al. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke 2004;35:1647–51. 10.1161/01.STR.0000131269.69502.d9 [DOI] [PubMed] [Google Scholar]
- 21. Koudstaal PJ, van Gijn J, Klootwijk AP, et al. Holter monitoring in patients with transient and focal ischemic attacks of the brain. Stroke 1986;17:192–5. 10.1161/01.STR.17.2.192 [DOI] [PubMed] [Google Scholar]
- 22. Hornig CR, Haberbosch W, Lammers C, et al. Specific cardiological evaluation after focal cerebral ischemia. Acta Neurol Scand 1996;93:297–302. 10.1111/j.1600-0404.1996.tb00524.x [DOI] [PubMed] [Google Scholar]
- 23. Rizos T, Güntner J, Jenetzky E, et al. Continuous stroke unit electrocardiographic monitoring versus 24-hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke 2012;43:2689–94. 10.1161/STROKEAHA.112.654954 [DOI] [PubMed] [Google Scholar]
- 24. Schuchert A, Behrens G, Meinertz T. Impact of long-term ECG recording on the detection of paroxysmal atrial fibrillation in patients after an acute ischemic stroke. Pacing Clin Electrophysiol 1999;22:1082–4. 10.1111/j.1540-8159.1999.tb00574.x [DOI] [PubMed] [Google Scholar]
- 25. Schaer B, Sticherling C, Lyrer P, et al. Cardiological diagnostic work-up in stroke patients--a comprehensive study of test results and therapeutic implications. Eur J Neurol 2009;16:268–73. 10.1111/j.1468-1331.2008.02413.x [DOI] [PubMed] [Google Scholar]
- 26. Schaer BA, Zellweger MJ, Cron TA, et al. Value of routine holter monitoring for the detection of paroxysmal atrial fibrillation in patients with cerebral ischemic events. Stroke 2004;35:68e–70. 10.1161/01.STR.0000117568.07678.4B [DOI] [PubMed] [Google Scholar]
- 27. Shafqat S, Kelly PJ, Furie KL. Holter monitoring in the diagnosis of stroke mechanism. Intern Med J 2004;34:305–9. 10.1111/j.1444-0903.2004.00589.x [DOI] [PubMed] [Google Scholar]
- 28. Lazzaro MA, Krishnan K, Prabhakaran S. Detection of atrial fibrillation with concurrent holter monitoring and continuous cardiac telemetry following ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2012;21:89–93. 10.1016/j.jstrokecerebrovasdis.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 29. Grond M, Jauss M, Hamann G, et al. Improved detection of silent atrial fibrillation using 72-hour Holter ECG in patients with ischemic stroke. Stroke 2013;44:3357–64. [DOI] [PubMed] [Google Scholar]
- 30. Stahrenberg R, Weber-Krüger M, Seegers J, et al. Enhanced detection of paroxysmal atrial fibrillation by early and prolonged continuous holter monitoring in patients with cerebral ischemia presenting in sinus rhythm. Stroke 2010;41:2884–8. 10.1161/STROKEAHA.110.591958 [DOI] [PubMed] [Google Scholar]
- 31. Ritter MA, Kochhäuser S, Duning T, et al. , 2013. Stroke Occult atrial fibrillation in cryptogenic stroke: detection by 7-day electrocardiogram versus implantable cardiac monitors;44:1449–52. 10.1161/STROKEAHA.111.676189 [DOI] [PubMed] [Google Scholar]
- 32. Higgins P, MacFarlane PW, Dawson J, et al. , 2013. Stroke Noninvasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke: a randomized, controlled trial;44:2525–31. 10.1161/STROKEAHA.113.001927 [DOI] [PubMed] [Google Scholar]
- 33. Thakkar S, Bagarhatta R. Detection of paroxysmal atrial fibrillation or flutter in patients with acute ischemic stroke or transient ischemic attack by holter monitoring. Indian Heart J 2014;66:188–92. 10.1016/j.ihj.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wachter R, Gröschel K, Gelbrich G, et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol 2017;16:282–90. 10.1016/S1474-4422(17)30002-9 [DOI] [PubMed] [Google Scholar]
- 35. Gumbinger C, Krumsdorf U, Veltkamp R, et al. , 2012. Eur J Neurol Continuous monitoring versus HOLTER ECG for detection of atrial fibrillation in patients with stroke;19:253–7. 10.1111/j.1468-1331.2011.03519.x [DOI] [PubMed] [Google Scholar]
- 36. Alhadramy O, Jeerakathil TJ, Majumdar SR, et al. Prevalence and predictors of paroxysmal atrial fibrillation on Holter monitor in patients with stroke or transient ischemic attack. Stroke 2010;41:2596–600. 10.1161/STROKEAHA.109.570382 [DOI] [PubMed] [Google Scholar]
- 37. Dangayach NS, Kane K, Moonis M. Paroxysmal atrial fibrillation in cryptogenic stroke. Ther Clin Risk Manag 2011;7:33–7. 10.2147/TCRM.S15079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gunalp M, Atalar E, Coskun F, et al. Holter monitoring for 24 hours in patients with thromboembolic stroke and sinus rhythm diagnosed in the emergency department. Adv Ther 2006;23:854–60. 10.1007/BF02850206 [DOI] [PubMed] [Google Scholar]
- 39. Fonseca AC, Brito D, Pinho e Melo T, et al. N-terminal pro-brain natriuretic peptide shows diagnostic accuracy for detecting atrial fibrillation in cryptogenic stroke patients. Int J Stroke 2014;9:419–25. 10.1111/ijs.12126 [DOI] [PubMed] [Google Scholar]
- 40. Manina G, Agnelli G, Becattini C, et al. , 2014. Intern Emerg Med 96 hours ECG monitoring for patients with ischemic cryptogenic stroke or transient ischaemic attack;9:65–7. 10.1007/s11739-012-0755-3 [DOI] [PubMed] [Google Scholar]
- 41. Tagawa M, Takeuchi S, Chinushi M, et al. Evaluating patients with acute ischemic stroke with special reference to newly developed atrial fibrillation in cerebral embolism. Pacing Clin Electrophysiol 2007;30:1121–8. 10.1111/j.1540-8159.2007.00823.x [DOI] [PubMed] [Google Scholar]
- 42. Shibazaki K, Kimura K, Fujii S, et al. Brain natriuretic peptide levels as a predictor for new atrial fibrillation during hospitalization in patients with acute ischemic stroke. Am J Cardiol 2012;109:1303–7. 10.1016/j.amjcard.2011.12.022 [DOI] [PubMed] [Google Scholar]
- 43. Vandenbroucke E, Thijs VN. Diagnostic and therapeutic impact of ambulatory electrocardiography in acute stroke. Acta Neurol Belg 2004;104:27–31. [PubMed] [Google Scholar]
- 44. Yodogawa K, Seino Y, Ohara T, et al. Prediction of atrial fibrillation after ischemic stroke using P-wave signal averaged electrocardiography. J Cardiol 2013;61:49–52. 10.1016/j.jjcc.2012.08.013 [DOI] [PubMed] [Google Scholar]
- 45. Atmuri K, Hughes A, Coles D, et al. The role of cardiac disease parameters in predicting the results of Holter monitoring in patients with acute ischaemic stroke. J Clin Neurosci 2012;19:965–8. 10.1016/j.jocn.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 46. Salvatori V, Becattini C, Laureti S, et al. Holter monitoring to detect silent atrial fibrillation in high-risk subjects: The perugia general practitioner study. Intern Emerg Med 2015;10:595–601. 10.1007/s11739-015-1241-5 [DOI] [PubMed] [Google Scholar]
- 47. Beaulieu-Boire I, Leblanc N, Berger L, et al. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis 2013;22:978–83. 10.1016/j.jstrokecerebrovasdis.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 48. Dogan U, Dogan EA, Tekinalp M, et al. P-wave dispersion for predicting paroxysmal atrial fibrillation in acute ischemic stroke. Int J Med Sci 2012;9:108–14. 10.7150/ijms.9.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Douen AG, Pageau N, Medic S. Serial electrocardiographic assessments significantly improve detection of atrial fibrillation 2.6-fold in patients with acute stroke. Stroke 2008;39:480–2. 10.1161/STROKEAHA.107.492595 [DOI] [PubMed] [Google Scholar]
- 50. Suissa L, Lachaud S, Mahagne MH, et al. Optimal timing and duration of continuous electrocardiographic monitoring for detecting atrial fibrillation in stroke patients. J Stroke Cerebrovasc Dis 2013;22:991–5. 10.1016/j.jstrokecerebrovasdis.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 51. Wohlfahrt J, Stahrenberg R, Weber-Krüger M, et al. , 2014. Eur J Neurol Clinical predictors to identify paroxysmal atrial fibrillation after ischaemic stroke;21:21–7. 10.1111/ene.12198 [DOI] [PubMed] [Google Scholar]
- 52. Lowres N, Neubeck L, Salkeld G, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost 2014;111:1167–76. 10.1160/TH14-03-0231 [DOI] [PubMed] [Google Scholar]
- 53. Svennberg E, Engdahl J, Al-Khalili F, et al. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176–84. 10.1161/CIRCULATIONAHA.114.014343 [DOI] [PubMed] [Google Scholar]
- 54. Proietti M, Mairesse GH, Goethals P, et al. A population screening programme for atrial fibrillation: a report from the Belgian Heart Rhythm Week screening programme. Europace 2016;18:euw069–86. 10.1093/europace/euw069 [DOI] [PubMed] [Google Scholar]
- 55. Kaasenbrood F, Hollander M, Rutten FH, et al. Yield of screening for atrial fibrillation in primary care with a hand-held, single-lead electrocardiogram device during influenza vaccination. Europace 2016;18:1514–20. 10.1093/europace/euv426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Engdahl J, Andersson L, Mirskaya M, et al. Stepwise screening of atrial fibrillation in a 75-year-old populationclinical perspective. Circulation 2013;127:930–7. [DOI] [PubMed] [Google Scholar]
- 57. Hendrikx T, Hörnsten R, Rosenqvist M, et al. Screening for atrial fibrillation with baseline and intermittent ECG recording in an out-of-hospital population. BMC Cardiovasc Disord 2013;13:41 10.1186/1471-2261-13-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hendrikx T, Sundqvist M, Sandström H, et al. Atrial fibrillation among patients under investigation for suspected obstructive sleep apnea. PLoS One 2017;12:e0171575 10.1371/journal.pone.0171575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chan NY, Choy CC. Screening for atrial fibrillation in 13 122 Hong Kong citizens with smartphone electrocardiogram. Heart 2017;103:24–31. 10.1136/heartjnl-2016-309993 [DOI] [PubMed] [Google Scholar]
- 60. Ramkumar S, Yang H, Wang Y, et al. The role of left atrial and ventricular function in prediction of atrial fibrillation: P2-19. Journal of the American Society of Echocardiography 2017;30:B70. [DOI] [PubMed] [Google Scholar]
- 61. Claes N, Goethals M, Goethals P, et al. Screening for atrial fibrillation (AF) in Belgium: a multicentre trial. European Journal of Cardiovascular Prevention and Rehabilitation 2011;1:S30. [Google Scholar]
- 62. Samol A, Masin M, Gellner R, et al. Prevalence of unknown atrial fibrillation in patients with risk factors. Europace 2013;15:657–62. 10.1093/europace/eus366 [DOI] [PubMed] [Google Scholar]
- 63. Battipaglia I, Gilbert K, Hogarth AJ, et al. Screening for atrial fibrillation in the community using a novel ECG recorder. J Atr Fibrillation 2016;9:29–31. 10.4022/jafib.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the alivecor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017;136:1784–94. 117.030583 10.1161/CIRCULATIONAHA.117.030583 [DOI] [PubMed] [Google Scholar]
- 65. Chan NY, Siu CW, Choy CC, et al. Effectiveness of community atrial fibrillation screening in over 10,000 citizens using smartphone electrocardiogram- The AFinder program ESC Congress. Barcelona, 2017. [Google Scholar]
- 66. Doliwa Sobocinski P, Anggårdh Rooth E, Frykman Kull V, et al. Improved screening for silent atrial fibrillation after ischaemic stroke. Europace 2012;14:1112–6. 10.1093/europace/eur431 [DOI] [PubMed] [Google Scholar]
- 67. Hendrikx T, Rosenqvist M, Wester P, et al. Intermittent short ECG recording is more effective than 24-hour Holter ECG in detection of arrhythmias. BMC Cardiovasc Disord 2014;14:41 10.1186/1471-2261-14-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stewart S, Murphy NF, Murphy N, et al. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004;90:286–92. 10.1136/hrt.2002.008748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. 10.1056/NEJMoa1105575 [DOI] [PubMed] [Google Scholar]
- 70. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
- 71. Desteghe L, Raymaekers Z, Lutin M, et al. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2017;19 euw025 10.1093/europace/euw025 [DOI] [PubMed] [Google Scholar]
- 72. Williams J, Pearce K, Benett I, et al. The effectiveness of a mobile ECG device in identifying AF: sensitivity, specificity and predictive value. British Journal of Cardiology 2015;22. [Google Scholar]
- 73. Ben Freedman S, Lowres N. Asymptomatic atrial fibrillation: the case for screening to prevent stroke. JAMA 2015;314:1911–2. 10.1001/jama.2015.9846 [DOI] [PubMed] [Google Scholar]
- 74. Aronsson M, Svennberg E, Rosenqvist M, et al. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace 2015;17:1023–9. 10.1093/europace/euv083 [DOI] [PubMed] [Google Scholar]
- 75. Jacobs MS, Kaasenbrood F, Postma MJ, et al. Cost-effectiveness of screening for atrial fibrillation in primary care with a handheld, single-lead electrocardiogram device in the Netherlands. Europace 2018;20:euw285 euw285 10.1093/europace/euw285 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-024178supp001.pdf (338.7KB, pdf)