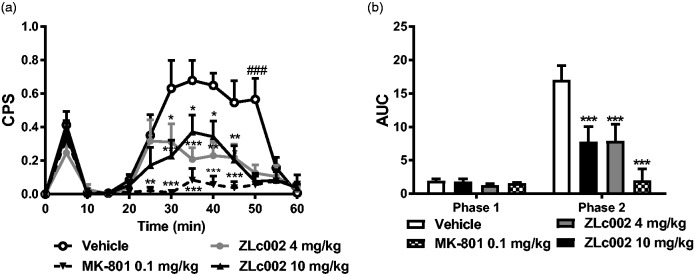
Figure 5.
ZLc002 reduces formalin-evoked pain behavior and Fos-like immunoreactivity in spinal dorsal horn. (a) ZLc002 (4 and 10 mg/kg, i.p.) reduced composite pain scores (CPS) from 30 to 50 min postformalin relative to vehicle. MK-801 (0.1 mg/kg) reduced CPS from 25 to 50 min postformalin relative to vehicle. (b) ZLc002 (4 and 10 mg/kg i.p.) (p < 0.001) and MK-801 (0.1 mg/kg i.p.) reduced formalin-evoked AUC of pain behavior scores relative to vehicle in phase 2 but not in phase 1 of formalin-evoked pain behavior. Data are mean ± S.E.M. (n = 5–6 per group) ***p < 0.001; **p < 0.01; *p < 0.05 vs. vehicle; ###p < 0.001 vs. all other groups, (two-way ANOVA followed by Bonferroni’s multiple comparison test). MK-801 and vehicle groups were published previously but run and processed concurrently with ZLc002-treated groups shown here (see methods and Carey et al.6). CPS: composite pain score; AUC: area under the curve.